
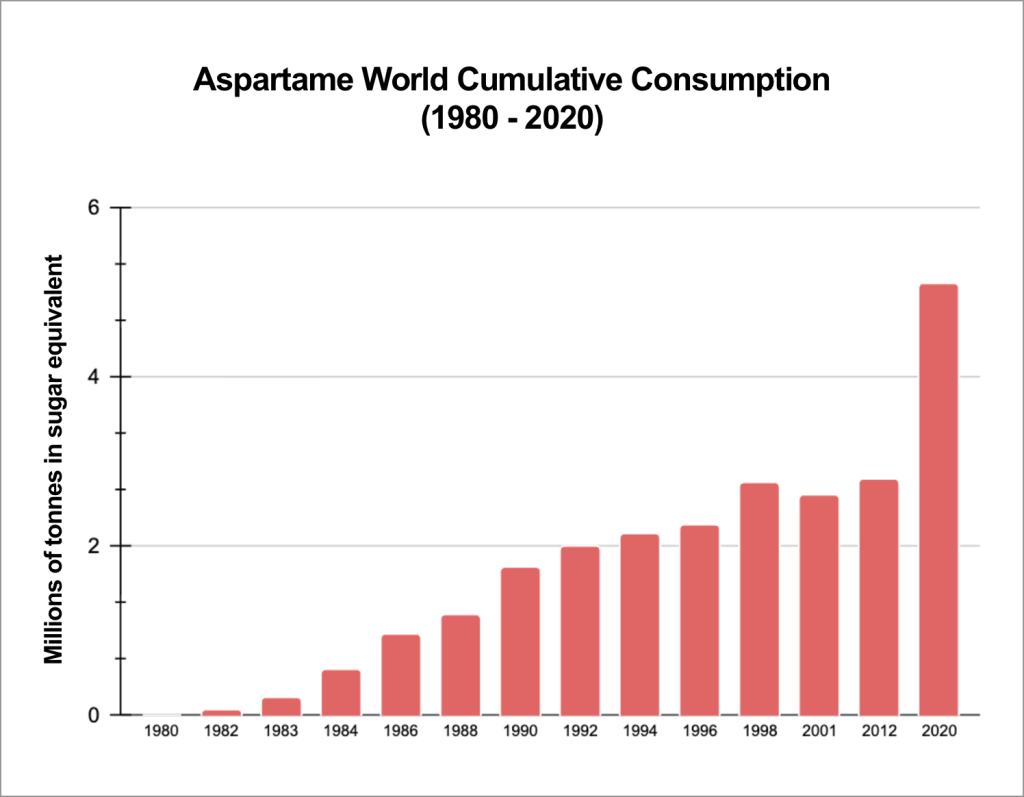
Aspartame is a synthetic sweetener with a sweetening power 150 to 200 times greater than that of sugar. It is used to provide a sweet taste without adding calories.
It can be found in more than 2,500 products in Europe, and about 6,000 worldwide, mainly in so-called “light”, “zero“, or “sugar-reduced” products1,2.
It represents a major economic factor for manufacturers since it is extremely profitable: its sweetening power allows for the use of tiny quantities, at a cost about ten times lower than that of sugar3. The savings made by producers are considerable but rarely passed on to selling prices. Regular drinks and their sugar-free versions are therefore often sold at the same price, or even more expensive in some cases, allowing margins to be maximized.
But the safety of aspartame is now widely questioned. Many scientists and investigative journalists denounce toxic effects downplayed by biased studies, as well as conflicts of interest between industry and health authorities that led to its authorization.
In this article, we shed light on the grey areas surrounding aspartame and take stock of what science really says.
A highly controversial market launch
1965: the birth of a sweetener under influence
Aspartame was discovered by chance in 1965 by a chemist working for the American pharmaceutical company Searle. This chemist, named James Schlatter, was developing a drug for ulcers when he synthesized a new molecule. Upon accidentally tasting the compound on his fingers, he noticed its extremely sweet taste4.
Its sweetening power, far greater than sugar, quickly caught the attention of the company, which then initiated an authorization process with the FDA (Food and Drug Administration) to allow its use in common food products.
Searle subsequently funded several laboratory tests to evaluate the toxicity of aspartame and submitted the results to the FDA in 1973. Following this, in 1974, the FDA approved its market release in dry foods and chewing gums. This decision was based on a preliminary, superficial reading of toxicity studies provided by Searle, and on the simplistic hypothesis that aspartame would be harmless since it breaks down in the body into two compounds that occur naturally in the human body5.
This authorization immediately sparked criticism, notably from neuroscientist John Olney, who warned of the risk of brain lesions and tumors6. The following year, in 1975, the market approval of aspartame was suspended to allow for further investigation by FDA scientists7.
After two years of work, the latter raised serious concerns. They identified numerous instances of negligence and irregularities in the studies conducted on rats and mice: unreported symptoms requiring antibiotics, tumor removal before dissection without mention in the reports, or the inability to examine certain organs that were too deteriorated8,9. In total, 52 major anomalies were found across just three of the fifteen toxicological studies provided by Searle10,11. Due to the seriousness of the findings, the FDA’s chief legal counsel initiated legal proceedings against Searle for “withholding data and false statements”12,13. The FDA also commissioned an external scientific panel to assess aspartame’s safety. This was the first time the FDA had used this process, known as a Public Board of Inquiry, to resolve a health safety issue14. In 1980, after several months of investigation, the panel unanimously concluded against the reauthorization of aspartame15.
The election of Ronald Reagan as President of the United States in 1980, however, marked a turning point in the history of aspartame. Donald Rumsfeld, then CEO of Searle, was no stranger to Washington: he had already held senior positions in the administration, notably as U.S. Ambassador to NATO and White House Chief of Staff under President Gerald Ford9.

After leaving politics to join Searle, he became actively involved in Reagan’s presidential campaign. After Reagan’s victory, Rumsfeld returned to government and joined the Interim Foreign Policy Advisory Committee. He then played a key role in nominating the new head of the FDA16. Arthur Hayes, a close associate of Rumsfeld with no experience in food additives, was thus appointed to lead the agency. Less than two months after taking office, in July 1981, Hayes reauthorized aspartame17—first for use in dry foods, then in beverages, before extending it to all food products in 1996. He stated: “I’m not prepared to say there is no risk from aspartame, but I thought it had been demonstrated that there was not a significant risk.”18

This decision goes against the conclusions of FDA toxicologists and independent experts from the Public Board of Inquiry19. Even more surprisingly, it also contradicted the stance of the main U.S. beverage lobby, the National Soft Drink Association. This organization had opposed the use of aspartame in sodas, arguing that the substance could degrade into toxic compounds during storage. In 1983, it stated: “Searle has not provided responsible certainty that aspartame and its degradation products are safe for use in soft drinks.”20
To legitimize the reauthorization of aspartame in the United States, two new studies were presented21,22. Both were conducted and funded by Ajinomoto, one of the world’s main… aspartame manufacturers! These studies, focused on cancer risks and other long-term toxic effects of aspartame, concluded there was no toxicity. However, like the previous studies conducted by Searle, Ajinomoto’s research fell far short of being unanimously accepted. First, they had never been submitted for peer review — a fundamental step to ensure a study’s scientific validity23. Moreover, their methodology did not meet the toxicological standards in place at the time24,25.
Furthermore, the 1981 cancer study conducted by Ajinomoto21 — which was used to justify reauthorization — was limited exclusively to brain tumors, thus ignoring the possibility that other organs might also be affected by a cancer risk. This approach had previously been deemed insufficient from a toxicological standpoint by the FDA’s internal scientists at the time26 to justify a market authorization.
Troubling ties between the FDA and aspartame manufacturers
Beyond relying on contested studies, the approval of aspartame also raises the issue of conflicts of interest. And for good reason: six senior FDA officials involved in the 1981 final approval of the sweetener later joined companies linked to the production… of aspartame!27
Another troubling fact: Samuel Skinner, the federal prosecutor in charge of leading the FDA’s legal action against Searle for irregularities in its studies, resigned on July 1st, 1977. Yet only a few months remained to act — until December 1977 — before the statute of limitations would make any action impossible. Shortly afterward, Skinner joined the law firm… that was defending Searle in the case! His replacement, William Conlon, arrived too late and didn’t have time to take over the case. Result: the charges were dropped, and Searle permanently escaped any sanctions. Strangely, Conlon ended up joining the same law firm defending Searle less than a year later12,28.
Another example of conflicts of interest: an investigation revealed that Arthur Hayes, appointed head of the FDA in 1981, accepted several perks offered by industry players29, including access to a private jet owned by General Food Corporation, a distributor of aspartame18. These revelations are said to have contributed to his resignation in 198330. Shortly after, he joined Burson-Marsteller, Searle’s public relations agency, as a senior scientific consultant13.
Europe: EFSA’s review also raises doubts
Despite the many controversies in the United States, the momentum was underway. Other countries began authorizing aspartame in everyday products, relying on the U.S. approval and without requiring additional studies: Canada in 198131, Australia in 198632, and then the European Union in 199433.
A widely criticized re-evaluation in 2013
In 2013, the European Food Safety Authority (EFSA) re-evaluated the safety of aspartame for its use in the European Union and concluded that there was no danger34,35. But this opinion was subsequently met with criticism. In 2019, Erik Millstone and Elizabeth Dawson, two researchers from the University of Sussex in the UK, published a study that questioned this assessment36. They raised concerns about how EFSA selected toxicological studies.
Indeed, they pointed out that each of the 73 studies showing harmful effects of aspartame on health had been excluded without solid justification. Conversely, 43 of the 62 studies that showed little or no impact from aspartame were included in the assessment — either partially or fully. Yet some of the excluded studies were considered by the two experts to be “more robust and more relevant” than those that were kept. Another issue: among the studies included, many were industry-funded, and some had even been described by former FDA toxicologists as “worthless,” “fraudulent,” or even a “disaster”37.
In fact, relying on industry-funded studies can introduce bias into the results. That’s the conclusion of two systematic reviews published in 201638 and 201739: studies funded by the food industry were more likely to conclude that sweeteners had no adverse health effects (notably on weight gain) compared to studies conducted independently.
The Ramazzini studies: benchmark research nevertheless dismissed by EFSA
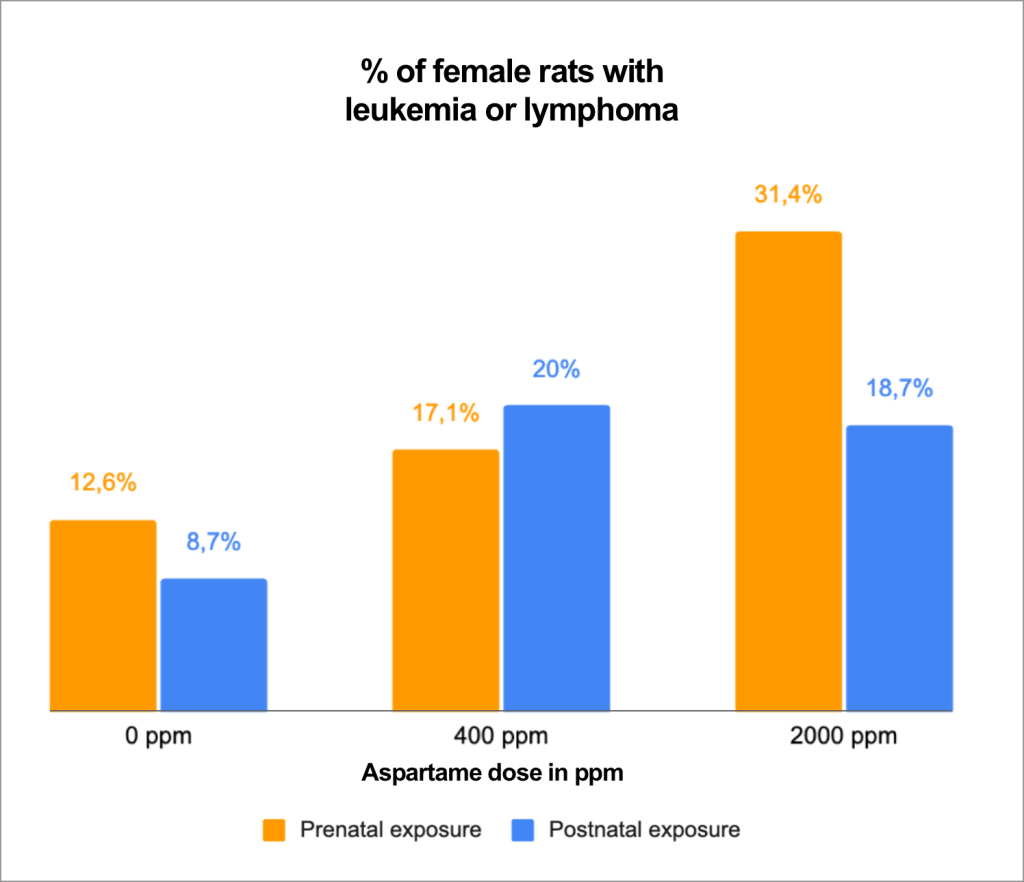
EFSA notably excluded several major studies40,41,42 from its re-evaluation, which addressed cancer risks and were conducted by the Ramazzini Institute, a research center internationally recognized in oncology43. EFSA justified its decision by arguing that the studies did not follow standard experimental protocols. Yet, these studies went beyond usual requirements: the study involved 1,400 rats (compared to 400 in standard protocols), tested six dose levels (instead of the usual three), and allowed the animals to live until their natural death to detect any late-onset carcinogenic effects — while standard protocols call for euthanizing rodents after about two years44.
EFSA’s main criticism focused on the unusual longevity of the rodents: according to the agency, lung infections observed in older animals made “the interpretation of results difficult.” EFSA thus suggested that tumors could have been caused by pulmonary infections rather than aspartame consumption34,45.
However, according to Ramazzini Institute researchers, these infections are part of the “ natural dying process in rodents”46. Moreover, such infections were also found in the control group, which was not exposed to aspartame, thus ruling out their involvement in tumor development. The presence of a control group was intended precisely to eliminate this type of bias, ensuring rigorous comparison.

The quality of Ramazzini Institute research on aspartame has since been recognized on several occasions. A paper published in 2008 by three American scientists — experts in experimental methods for assessing the carcinogenicity of substances — suggested that allowing animals to live longer helped “strengthen the value and validity of results for regulatory agencies”47. As researcher Erik Millstone reiterated in an interview, “The Ramazzini studies were more thorough, sensitive, reliable and relevant to human exposure than those conducted in accordance with conventional protocols.”
In 2021, the Ramazzini Institute published a new study confirming that the tumors observed in rodents in its early studies were indeed linked to aspartame consumption, and not to lung infections that naturally appear at the end of life48. In 2023, the main controversial study was reanalyzed by new researchers from the Ramazzini Institute, and the conclusions remained unchanged49.
Finally, the International Agency for Research on Cancer (IARC) — a global reference in cancer research — confirmed the robustness of the Ramazzini Institute’s methodology in 2024. It stated that allowing rats to live longer was one of the study’s main strengths. It also emphasized that the purity of the aspartame used had been correctly analyzed. Conversely, the IARC found the studies by Searle and Ajinomoto limited due to the lack of purity data and insufficient histopathological detail50.
By refusing to include Ramazzini’s data, EFSA dismissed some of the strongest available evidence regarding cancer risks, raising questions about its impartiality. The Ramazzini Institute later revealed in 2021 that it had been under intense pressure after publishing its findings on aspartame. The researchers behind these studies had their credibility attacked, and their work was mocked by the agri-food industry51,52,53, leading to a significant reduction in their funding54.
The Center for Science in the Public Interest (CSPI), a U.S. NGO recognized for its work on public health, also denounced these attacks against the Ramazzini Institute back in 201355. The organization reiterated that the institute’s research provides “unequivocal evidence that aspartame is carcinogenic in animals.”
EFSA also suspected of conflict of interest
In their 2019 study36on EFSA’s re-evaluation of aspartame, researchers at the University of Sussex suggested that the agency may have been influenced by commercial conflicts of interest. According to them, this could explain why some otherwise robust studies were excluded from the 2013 EFSA review.
This hypothesis is consistent with findings from the NGO Foodwatch. In a report on aspartame published in 202556, the organization stated that, among the 62 studies deemed reliable by EFSA, 45 showed conflicts of interest: they were directly or indirectly linked to aspartame manufacturers, which could have influenced the agency’s assessment.
The analysis published by Réseau Environnement Santé in 201357 went even further, revealing that 6 of the 19 EFSA experts who participated in the re-evaluation of aspartame had close ties to industry — notably with Ajinomoto, the main global manufacturer of aspartame, and with Coca-Cola, whose “light” and “zero” products heavily rely on the sweetener. More concerning still, this analysis found that part of the EFSA’s 2013 report was essentially a copy-paste of a 2007 publication funded by Ajinomoto51. Of the 96 lines in the EFSA report relating to neurological risks linked to aspartame, 60 were identical or very close to the wording of the Ajinomoto-funded publication. In its report, Réseau Environnement Santé concluded that the 2013 EFSA re-evaluation shows serious shortcomings with respect to the core principles of scientific ethics.
EFSA also appears to have attempted to downplay — or even hide — certain links between its members and industry. This was revealed by investigative journalist Marie-Monique Robin in her documentary Aspartame: Our Daily Poison58, broadcast in 2011 on Arte. It shows that the agency later altered an official conflict-of-interest declaration. The case involved Dominique Parent-Massin, a member of the EFSA working group tasked with evaluating aspartame. In her initial file, this EFSA expert stated that she had worked as a consultant for both Ajinomoto and Coca-Cola — two companies directly involved with the sweetener. Yet these disclosures reportedly disappeared from her record after the journalist interviewed EFSA staff during her investigation. This change erased all traces of her direct ties with EFSA and two companies that had a major commercial interest in aspartame, raising fundamental concerns about transparency and independence.
These revelations are in line with those from the NGO Corporate Europe Observatory. In a 2011 report59, the organization explained that four members of the EFSA panel evaluating food additives had failed to disclose their current and past collaborations with the International Life Sciences Institute (ILSI Europe). Though officially presented as a non-profit scientific organization, ILSI has long defended the interests of the food industry60. It was founded by a former Coca-Cola vice-president, financed for decades by Coca-Cola (until 2021) and Mars (until 2018), and currently counts Pepsi and Ajinomoto among its members. The NGO’s report concludes that EFSA urgently needs to adopt stricter rules on conflicts of interest and undergo major reform to regain public trust.
Moreover, the European Parliament strongly questioned the independence of EFSA in a report published in 201461,62, calling for a real transparency and conflict-of-interest prevention policy. In response, the agency took several measures: in 201463 it introduced rules to prevent conflicts of interest among its members. In 201564, it revised its methodology for selecting studies to avoid arbitrarily excluding certain findings. Finally, in 201965, a European regulation was adopted to enhance EFSA’s transparency. However, despite these improvements, the 2013 opinion on aspartame has still not been updated.In light of all these revelations, several scientists are now calling for an independent re-evaluation of aspartame at the European level36,48,66,67,68.
Well-documented risks
Cancer: an increased risk from half a can per day
In 2023, the International Agency for Research on Cancer (IARC) classified aspartame as “possibly carcinogenic” (Group 2B)69. This decision was based on a comprehensive synthesis by IARC, which reviewed all studies published since 1974 on the carcinogenic potential of this sweetener. In a 471-page report70, IARC concluded that aspartame is possibly carcinogenic to humans.
In addition to animal studies, this classification also relies on human data — notably from the prestigious French cohort NutriNet-Santé, led by the French National Institute of Health and Medical Research (INSERM) in 202271. This large-scale study has been tracking the dietary habits of over 100,000 French adults for 13 years. It showed that regular consumption of aspartame was associated with a 15% increase in overall cancer risk, with an even higher risk for breast cancer, where the increase reached up to 22%. People who consumed aspartame regularly, equivalent to half a can of soda per day, were more likely to develop these cancers. This is the first study in the world to demonstrate such a precise and rigorously verified association between aspartame exposure and cancer risk. The IARC considers it “the most detailed” and “the most comprehensive study” on human exposure to this sweetener.
A previous epidemiological study conducted by Harvard University and published in 201272 followed more than 120,000 Americans over 22 years and also found a statistically significant association between the consumption of beverages sweetened with aspartame and a 31% increase in the risk of non-Hodgkin lymphoma, and a 102% increase in the risk of multiple myeloma.
According to some members of the IARC expert group, aspartame should even have been placed in the higher category as a “probable carcinogen” (Group 2A). In their view, the Ramazzini Institute provided sufficient evidence of carcinogenicity in animals70. The researchers at the Ramazzini Institute also disputed the way their data was handled by the IARC. According to them, the IARC underestimated their results. The researchers conclude that the IARC’s evaluation of aspartame “may have been guided by factors other than dispassionate scientific evaluation of the available data”, suggesting a possible conflict of interest within the IARC working group73.
Numerous studies on human and animal cells have identified several mechanisms that could explain tumor development. According to the IARC, the most likely cause is oxidative stress. When digested, aspartame breaks down into substances that can generate free radicals — unstable molecules that, in large quantities, can oxidize — that is, damage — cells. Over time, this cellular damage could impair proper function and increase cancer risk70.
Today, the suspicion of aspartame’s carcinogenicity is based on a body of evidence from three complementary approaches:
- First, animal studies conducted by the Ramazzini Institute40,41,42, whose more demanding protocols compared to usual standards, produced results that have been widely recognized by the scientific community.
- Second, epidemiological studies in humans, including one considered by IARC to be the most complete and rigorous on the subject71, have demonstrated a significant association between aspartame consumption and increased cancer risk.
- Third, mechanistic research has identified a coherent mode of action explaining how tumors develop70.
The convergence of these findings, coming from various disciplines and methods, forms a body of evidence robust enough for IARC to classify aspartame as “possibly carcinogenic to humans“.
Not surprisingly, the few studies that concluded aspartame poses no cancer risk are often funded by industry. Moreover, they frequently exhibit major limitations. Some are based on outdated methodologies, like those used by Searle and Ajinomoto in the 1980s21,22. Others use genetically modified mice, a model the IARC considers inadequate, since such animals may be insensitive to aspartame70. Finally, some studies focus on just one specific type of cancer, or use observation periods that are too short to detect long-term effects. As a result, the scientific quality of these studies is far inferior to those conducted independently and rigorously.
A sweetener that could promote diabetes
Like other artificial sweeteners, aspartame has for several years been suspected of contributing, in the long term, to the development of type 2 diabetes.
This chronic disease involves a progressive dysfunction of the body’s ability to regulate glucose (blood sugar) levels. It is the most common form of diabetes, affecting over 460 million people worldwide, around 6% of the global population. It typically develops in adulthood, influenced by lifestyle factors. The main known risk factors today are: sedentary lifestyle, unbalanced diet, and smoking74. However, in recent years, scientists have begun to suspect that certain sweeteners — especially aspartame — may contribute to the development of this disease.
Since 2008, twelve cohort studies recognized as robust by the WHO75 have shown that regular consumption of artificially sweetened beverages could increase the risk of developing type 2 diabetes. For instance, a French study published in 201376,involving 66,000 women, revealed that regular consumers of artificially sweetened drinks had up to twice the risk of developing type 2 diabetes compared to those who didn’t consume them. Surprisingly, in some cases, the risk was comparable to that observed among people who regularly consume sugary drinks, already known to promote diabetes. Comparable findings were observed in human studies published in 200977 and 201478, as well as in animal studies in 201779.
Three main mechanisms are currently considered to explain these findings:
- Sweeteners may maintain or reinforce cravings for sweet taste, encouraging higher consumption of sugary products80.
- Sweeteners, especially aspartame, may trigger physiological responses similar to those caused by sugar, by activating sweet taste receptors not only in the mouth but also in the gut and pancreas. A 2010 American study81 showed that aspartame consumption led to an increase in insulin levels comparable to that caused by sugar consumption 30 minutes after a meal.
- Lastly, sweeteners could disrupt the gut microbiota, leading to chronic low-grade inflammation, which promotes insulin resistance and increases the risk of type 2 diabetes82.
In 2023, a study from the French NutriNet-Santé cohort83 — praised for its methodological rigor — produced even more precise results. It revealed that regular consumption of even a small amount of aspartame could increase the risk of developing type 2 diabetes by 48% to 63%. The authors concluded that these sweeteners cannot be considered safe sugar substitutes, even when used in low doses.
Many other studies have also shown an association between sweetener consumption and diabetes84. A study published in 2022 even stated that sweeteners, especially aspartame, “may even contribute to the diabetes pandemic in some contexts”82.
Increased risk of cardiovascular disease
Aspartame is also suspected of increasing the risk of cardiovascular disease. These conditions — including heart attacks, strokes, venous thrombosis, and pulmonary embolisms — affect the heart and blood vessels, and are the leading cause of death worldwide85.
In April 2022, the World Health Organization (WHO) published a report on the effects of sweeteners75, based on 280 human studies. Of these, 56 focused specifically on cardiovascular disease. The synthesis revealed that regular sweetener consumers had a 32% higher risk of developing cardiovascular disease, and a 19% higher risk of stroke. Moreover, still according to WHO, regular sweetener consumption promotes hypertension and cholesterol imbalance, two key factors in the development of cardiovascular complications.
These results align with those of the French NutriNet-Santé study, published in September 202286, which specifically investigated the impact of aspartame on cardiovascular health. It revealed that regular consumers had a 17% higher risk of stroke, an estimate close to what was cited by the WHO..
In response to these alarming findings, the WHO issued a new guideline in 2023, stating clearly: “Do not consume sweeteners for the purpose of reducing the occurrence of chronic diseases, particularly cardiovascular disease.”87
A 2025 study88, using a recent protocol for analyzing chemical interactions, provides further insight into these observations. It suggests that aspartame may interact with several proteins and receptors in the brain, causing multiple interferences that promote hypertension and stroke risk. The researchers even suggested potential incompatibilities between daily aspartame consumption and the use of certain medications meant to lower blood pressure.
A harmful impact on the microbiota
The intestinal microbiota refers to the billions of micro-organisms that inhabit our intestines and play a crucial role in our health89. When disrupted, it may contribute to the development of numerous chronic diseases90,91,92,93 (see our article on the microbiota).
In 2014, a study published in the prestigious journal Nature was the first to reveal a negative effect of sweeteners on the microbiota of healthy individuals94. The researchers observed a decrease in microbial diversity. This phenomenon may contribute, over time, to metabolic disorders such as insulin resistance, glucose intolerance, and the chronic conditions already discussed: cancer, type 2 diabetes, and cardiovascular diseases.
In 2014, another study focused specifically on aspartame95. It showed that even at typical doses — equivalent to two cans of soda per day — aspartame can durably alter the balance of the intestinal microbiota.
Other research published in 202096 highlighted an aggravating factor: aspartame may damage the intestinal epithelium, the thin barrier of cells that protects us from pathogens. Its deterioration promotes chronic gut inflammation and weakens the immune system, increasing the risk of developing chronic diseases.
Further research is needed to better understand the link between sweeteners, microbiota, and chronic diseases. However, this hypothesis is now widely considered plausible by the scientific community97,98,99. In its 2022 report75, the WHO also noted that certain chronic conditions could be linked to microbiota imbalances caused by sweetener consumption.
A suspicion of neurotoxicity
The first warnings about the possible neurological toxicity of aspartame date back to the 1970s. At the time, neuroscientist John Olney observed that even at relatively low doses, the sweetener could destroy neurons in rodent brains still under development100,101. Since then, these concerns have resurfaced several times, fueled by new studies.
In 2008, researchers showed that aspartame breaks down in the body into three substances102: phenylalanine, aspartic acid, and methanol. All are known to interact with the nervous system. They can disrupt neurotransmitters like dopamine and serotonin, and may cause headaches, memory or sleep disorders, mood swings, stress, or depression4,103. Finally, they can generate oxidative stress in the brain, damaging neurons and promoting their degenerationn104.
Two studies from 2023105 and 2024106 showed that these effects occurred in mice at very low aspartame doses, comparable to daily human intake. These effects appeared in just four weeks and — more concerning — were passed on to the offspring, suggesting that aspartame’s effects on the brain could be transmitted across generations.
The WHO states that in humans, results remain mixed: some studies show a link between aspartame and cognitive disorders — including an increased risk of Alzheimer’s disease — while others find no association75. Further in-depth research is needed to determine whether the effects seen in animals also occur in humans.
An outdated and insufficient ADI
The ADI (Acceptable Daily Intake) refers to the maximum quantity of a substance that a person can consume every day over a lifetime without health risk. It is calculated based on body weight, and is expressed in milligrams (mg) of substance per kilogram (kg) of body weight107.
This limit is set by risk assessment agencies, such as EFSA in the EU or the FDA in the United States. To do so, they identify the lowest dose that causes harm in animal studies, then apply a safety factor (usually a factor of 100) to determine a supposed safe value for humans. While useful, this method nevertheless has certain limitations.
A threshold set by manufacturers in the 1970s
The current ADI (Acceptable Daily Intake) for aspartame is based on old and controversial studies. In 1980, the JECFA (the committee in charge of setting global ADIs) established this limit at 40 mg/kg of body weight per day108. This decision was largely influenced by the FDA, whose scientists also sat on JECFA109. Moreover, at the time, the FDA was the only agency in possession of all available toxicological data, but it did not allow JECFA to fully consider the fraud and methodological flaws of the studies in question. In fact, these issues are not mentioned in JECFA’s official reports.
To define this ADI, JECFA relied on studies funded by Ajinomoto and Searle. The key study used to set the ADI was conducted in 1981 on rats by Ajinomoto22 — a highly controversial study, whose methodology was already questioned by many scientists at the time12,110.
In 1983, the FDA adopted an even more lenient ADI of 50 mg/kg/day, without basing this decision on any new studies111.
In Europe, the EFSA also set the ADI at 40 mg/kg/day in 1984, when aspartame was approved for market use112. EFSA relied heavily on the FDA and JECFA’s assessments, without requesting additional studies58. In its report, EFSA admitted to having reviewed a very large amount of toxicological data without specifying which studies or results were considered. Later, during a hearing at the European Parliament in 2011, EFSA admitted that the scientific committee at the time had, in fact, never had access to the original studies from Searle and Ajinomoto, and had authorized aspartame throughout the European Union with its eyes closed112.
A status quo upheld in 2013 without justification
In 201334, EFSA reassessed aspartame and maintained the ADI value set in 1984: 40 mg/kg of body weight per day113. According to WHO estimates, this corresponds to a daily consumption of 9 to 14 cans of soda114,115 — an amount that may seem extreme, and thus falsely reassuring for the average soda drinker.
However, as previously mentioned, this re-evaluation has been widely criticized and raised a major issue: the biased selection of studies. EFSA tended to favor the studies most reassuring about aspartame, and to dismiss those that indicated risks — including ones with a methodology considered more robust than that of the studies it retained36,57.
The agency states that it relies on the highly controversial 1981 study funded by Ajinomoto, the manufacturer of aspartame22. Its report never mentions the major methodological flaws of the study. One of the main limitations of this study is regarding the absence of data on the purity of aspartame: today, nothing makes it possible to state with certainty that the substance administered to the rats in the study was indeed pure aspartame70. To dismiss this concern, EFSA simply states that in all long-term studies, “the purity of the aspartame used is considered to be 100%”34.
The credibility of the 2013 re-evaluation was also weakened by revelations about conflicts of interest involving some EFSA experts and the aspartame industry57,59,62. These concerns led to the adoption of a European law in 2019 aimed at strengthening transparency and scientific integrity within EFSA65,166. This was a step forward, though it never led to reopening the aspartame file or re-evaluating its ADI.
In 2023, JECFA in turn chose to maintain aspartame’s ADI unchanged114,117. Once again, this conclusion was largely based on the 1981 study funded by Ajinomoto118.
However, many stakeholders — including the European NGO Foodwatch119 and the American NGO U.S. Right to Know120 — pointed out similar problems at JECFA: biased selection of studies, the involvement of six committee members also working with ILSI, and major transparency issues regarding conflicts of interest118.
Major risks excluded from evaluation criteria
In addition to relying on old and controversial studies, the ADI for aspartame set in Europe is based on research that does not cover all the potential toxic effects of this substance.
To establish an ADI, health authorities rely exclusively on studies conducted on animals (rats, mice, or rabbits) exposed for two years to a substance whose composition and purity are assumed to be fully known. The only effects considered are those visible during biological assessments, such as: weight changes, anomalies in organ development or function, impacts on reproduction or fertility, effects on offspring, tumor formation, or other abnormalities.
However, these animal models do not always allow for the identification of all harmful effects in humans. Some impacts — on behavior, memory, appetite, or even so-called “cocktail effects” linked to simultaneous exposure to other common substances (drugs, additives, pesticides, etc.) — are difficult, if not impossible, to detect using this approach. This limitation likely leads to underestimating certain risks, such as the possible neurotoxicity of aspartame or the progressive development of insulin resistance. The WHO also pointed out, in the introduction of its 2022 report, that “the long-term health effects of consuming sweeteners at levels below the ADI are not well characterized.”
Moreover, human data is not considered when setting ADIs121. In fact, these thresholds are typically determined at the time a new substance receives market authorization. While clinical trials on humans are mandatory for medications, this is not the case for food additives. From an ethical standpoint, it would be hard to justify exposing a group of volunteers to a new molecule solely to validate its commercial approval, especially when a food additive does not offer the same therapeutic benefit as a drug. Human data is therefore analyzed only afterwards, mainly through epidemiological studies. These aim to determine, once the additive is already consumed, whether regular consumers show a higher frequency of certain illnesses than the general population. Several scientists have gone so far as to call the ADI a “makeshift procedure that relies as much on science as on assumptions”122 and have called for “a more rigorous approach based on human data”123.
Several epidemiological studies – conducted in humans – have highlighted harmful effects of aspartame at doses that are nonetheless authorized, commonly consumed, and well below the ADI. These studies, for example, show an increased risk of cancers124, cardiovascular diseases125, and type 2 diabetes126, at doses nearly 200 times lower than the ADI, i.e. the equivalent of half a can of soda per day. A higher risk of obesity127, as well as higher birth weight in newborns128, has also been observed in people who consume just one “sugar-free” soda per day. However, these studies are not included in the calculation of the ADI, as they do not allow for determining a no-risk dose, unlike animal studies.
Moreover, during EFSA’s 2013 re-evaluation34, animal studies on emerging risks — such as effects on the gut microbiota — were not taken into account. Yet these risks are now considered concerning, given the potentially serious consequences that a microbiota disruption linked to aspartame could cause129.
The current ADI threshold is therefore incomplete and does not fully protect public health, making the lack of revision of the ADI all the more concerning.
A sweetener with no benefit for weight control
Since its authorization, aspartame has been promoted as an alternative to sugar that helps reduce caloric intake and improve weight management. This argument may seem logical on paper.
However, an increasing number of studies are challenging this claim. In 2015, ANSES (the French Agency for Food, Environmental and Occupational Health & Safety) published a report based on 383 scientific publications130. It concluded that sweeteners, including aspartame, have shown no beneficial effect on weight loss or glycemic control. These findings also apply to people in good health as well as those with diabetes.
The conclusions of ANSES echo those of the WHO report published in 202275. In fact, short-term studies (less than 12 months) suggest a small effect on weight or waist circumference in individuals trying to lose weight131. However, over the longer term, cohort studies conducted over 4 to 9 years show the opposite trend: higher sweetener consumption is associated with a 2.6 cm increase in waist circumference132. The WHO even reports that regular sweetener consumption is associated with a 76% increase in the risk of obesity, as well as a significant rise in body mass index (BMI). In conclusion, the WHO explicitly recommends “not to use sweeteners for weight loss purposes.” In children, ANSES also notes that most long-term follow-up studies paradoxically show a link between regular sweetener consumption and weight gain133.
Even more concerning: a Canadian study from 2020128, conducted on more than 2,200 pregnant women, shows that babies exposed to aspartame in utero have a significantly higher birth weight compared to those not exposed. These babies are also at higher risk of metabolic complications, such as insulin resistance or fat accumulation in tissues — factors that increase their susceptibility to later weight gain and promote the development of obesity in childhood or adulthood.The exact mechanism behind this effect is still poorly understood. Several hypotheses have been proposed: inappropriate activation of sweet taste receptors, impaired glucose tolerance134, the progressive development of insulin resistance, or disruption of the gut microbiota135.
Conclusion
The precautionary principle lies at the heart of the European regulatory system, where it has been enshrined in law since 2002136. However, the history of aspartame shows how this principle can be weakened when risk assessment is drowned under the influence of industrial lobbies.
The question is no longer simply whether aspartame poses a health risk, but whether our authorities are still capable of effectively protecting consumers.
Faced with this situation, it is essential that a clear message be heard: economic interests must not dictate decisions affecting public health. This is the message of the European petition supported by Yuka, Foodwatch and the Ligue Contre le Cancer, which calls on the European Commission to ban aspartame. Health should never be a bargaining chip for economic interests.
- ¹ Valavanidis, 2023. Aspartame, Artificial Sweetener, Credible Evidence that is Potentially Carcinogenic? IARC will decide in 2023 if an increased risk of cancer is associated with Aspartame consumption. https://www.researchgate.net/profile/Athanasios-Valavanidis/publication/372344870_Aspartame_Artificial_Sweetener_Credible_Evidence_that_is_Potentially_Carcinogenic_IARC_will_decide_in_2023_if_an_increased_risk_of_cancer_is_associated_with_Aspartame_consumption/links/64b11080c41fb852dd6ff270/Aspartame-Artificial-Sweetener-Credible-Evidence-that-is-Potentially-Carcinogenic-IARC-will-decide-in-2023-if-an-increased-risk-of-cancer-is-associated-with-Aspartame-consumption.pdf
- ² Shaher et al, 2023. Aspartame Safety as a Food Sweetener and Related Health Hazards. https://www.mdpi.com/2072-6643/15/16/3627
- ³ Market prices: aspartame USD 10,49 per kg (https://www.imarcgroup.com/aspartame-pricing-report
- (Germany)), sugar USD 0,5 per kg (https://www.statista.com/statistics/675828/average-prices-sugar-worldwide/
- ). Achieving same sweetness (aspartame 200x that of sugar) USD 0,5 for sugar and USD 0,05 for aspartame needed (calculation made on December 6, 2024).
- ⁴ Czarnecka et al, 2021. Aspartame—True or False? Narrative Review of Safety Analysis of General Use in Products. Nutrients. 2021 Jun 7;13(6):1957. https://pmc.ncbi.nlm.nih.gov/articles/PMC8227014/
- ⁵ Food and Drug Administration, 1974. Rules and Regulations. Federal Register, vol. 39, no. 145 - Friday, July 26, 1974. https://archives.federalregister.gov/issue_slice/1974/7/26/27316-27320.pdf#page=2
- ⁶ Mission Possible, 2006. Report for schools, ob-gyn and pediatricians on children and aspartame/MSG. Prepared By Mission Possible, Dr. Betty Martini. https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=d0041fbf9e37d3c641bab2a336221cf41a9933bd
- ⁷ Food and Drug Administration, 1975. Rules and Regulations. Federal Register, vol. 40, no. 235 - Friday, December 5, 1975. https://archives.federalregister.gov/issue_slice/1975/12/5/56899-56910.pdf
- ⁸ Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment, 1990. Aspartame - Validity of Searle toxicity studies. TOX/90/44. https://data.parliament.uk/DepositedPapers/Files/DEP2013-0273/8TOX9044.pdf
- ⁹ Swankin and Turner. Aspartame / NutraSweet, The History of the Aspartame Controversy. http://www.swankin-turner.com/hist.html
- ¹⁰ The Guardian, 2005. Safety of artificial sweetener called into question by MP. https://www.theguardian.com/politics/2005/dec/15/foodanddrink.immigrationpolicy
- ¹¹ Blaylock, 2006. Aspartame Is An Excitoneurotoxic Carcinogenic Drug! https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=eef405cbc0079082949016cd279a13a542588958
- ¹² Mullarkey and Newman, 1994. Sweet Delusion. How safe is your artificial sweetener ? Part one: the hidden history of Aspartame. https://ia800109.us.archive.org/11/items/OnAspartameMsg/SweetDelusion.pdf
- ¹³ Broer, 2002. Aspartame: a chronicle of crime. Fit at forty-sixty and beyond. The in-depth health and nutrition analysis. Vol. 1, no 2, article 3. https://irp.cdn-website.com/6b820530/files/uploaded/Aspartame%20Dangers.pdf
- ¹⁴ Smyth, 1983. The FDA’s Public Board of Inquiry and the Aspartame Decision. Indiana Law Journal, vol. 58, issue 4. https://www.repository.law.indiana.edu/cgi/viewcontent.cgi?article=2268&context=ilj
- ¹⁵ Nill, 2000. The History of Aspartame. Harvard Library. https://dash.harvard.edu/server/api/core/bitstreams/7312037c-a98d-6bd4-e053-0100007fdf3b/content
- ¹⁶ UK Parliament. Artificial Sweeteners. 14 Dec 2005 : Column 491WH-continued. https://publications.parliament.uk/pa/cm200506/cmhansrd/vo051214/halltext/51214h05.htm
- ¹⁷ Food and Drug Administration, 1981. Federal Register, vol. 46, no. 142, Friday, July 24, 1981, Notices. https://archives.federalregister.gov/issue_slice/1981/7/24/38256-38289.pdf#page=30
- ¹⁸ Sykes, 2015. The Aspartame Controversy of 1981. The Hidden Truth Behind the Not-So-Sweet Artificial Sweetener. https://vtechworks.lib.vt.edu/server/api/core/bitstreams/a814bc16-5c18-4e53-82eb-6472e092e7af/content
- ¹⁹ Shapiro, 1986. Scientific Issues and the Function of Hearing Procedures: Evaluating the FDA’s Public Board of Inquiry. https://scholarship.law.duke.edu/cgi/viewcontent.cgi?article=2944&context=dlj
- ²⁰ Millstone, 2019. Aspartame Chronology. https://www.sussex.ac.uk/webteam/gateway/file.php?name=doc-3---chronology-metzenbaum-1986002.pdf&site=25
- ²¹ Ishii, 1981. Incidence of brain tumors in rats fed aspartame. Toxicology Letters. 1981 Mar;7(6):433-7.https://pubmed.ncbi.nlm.nih.gov/7245229/
- ²² Ishii et al, 1981. Toxicity of aspartame and its diketopiperazine for Wistar rats by dietary administration for 104 weeks. Toxicology, 1981;21(2):91-4. https://pubmed.ncbi.nlm.nih.gov/7281205/
- ²³ Soffritti et al, 2014. The Carcinogenic Effects of Aspartame: The Urgent Need for Regulatory Re-Evaluation. American Journal of industrial Medicine. 57:383–397 (2014). https://www.ramazzini.org/wp-content/uploads/2009/02/The-carcinogenic-effects-of-aspartame_The-urgent-need-for-regulatory-re-evaluation-2014.pdf
- ²⁴ National Cancer Institute, 1976. Guideline for Carcinogen Bioassay in Small Rodents. NCI-CG-TR-1. https://ntp.niehs.nih.gov/sites/default/files/ntp/htdocs/lt_rpts/tr001.pdf
- ²⁵ Jacobs and Hatfield, 2012. History of Chronic Toxicity and Animal Carcinogenicity Studies for Pharmaceuticals. Veterinary Pathology. 2012;50(2):324-333. https://journals.sagepub.com/doi/10.1177/0300985812450727
- ²⁶ Condon, 1981. Memorandum: Aspartame- Dissenting Opinion on the Brain Tumor Issue. May. 19, 1981. https://www.sussex.ac.uk/webteam/gateway/file.php?name=doc-18---condon-to-levitt-19may1981.pdf&site=25
- ²⁷ Government Accountability Office, 1986. Six Former HHS Employees’s Involvement in Aspartame’s Approval. Food and Drug Administration. Briefing report to the Honorable Howard Metzenbaum United State Senate. https://www.gao.gov/assets/hrd-86-109br.pdf
- ²⁸ The Ecologist, 2005. Aspartame. September 2005. https://h2rc2.com/Ecogypt/page1/page17/assets/AspartameResearch.pdf
- ²⁹ Huffpost, 2011. Donald Rumsfeld and the Strange History of Aspartame. https://www.huffpost.com/entry/donald-rumsfeld-and-the-s_b_805581
- ³⁰ Food and Drug Administration, 2020. Arthur Hull Hayes. https://www.fda.gov/about-fda/fda-leadership-1907-today/arthur-hayes
- ³¹ Government of Canada, 2023. L’aspartame. https://www.canada.ca/fr/sante-canada/services/aliments-nutrition/salubrite-aliments/additifs-alimentaires/succedanes-sucre/aspartame-edulcorants-artificiels.html
- ³² news.com.au. ‘Increased risk’: Warning over cult diet drinks after animal study. https://www.news.com.au/lifestyle/health/health-problems/warning-over-cult-diet-drink-revealed/news-story/58b3bea834a099dc9bf2fbef3d65d266
- ³³ European Food Safety Authority, 2011. EFSA publishes original industry studies on aspartame. https://www.efsa.europa.eu/en/press/news/111124-0
- ³⁴ European Food Safety Authority, 2013. Scientific Opinion on the re-evaluation of aspartame (E 951) as a food additive. EFSA Journal 2013;11(12):3496. https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2013.3496
- ³⁵ European Food Safety Authority, 2023. Aspartame. https://www.efsa.europa.eu/en/topics/topic/aspartame
- ³⁶ Millstone et Dawson, 2019. EFSA’s toxicological assessment of aspartame: was it even-handedly trying to identify possible unreliable positives and unreliable negatives? Arch Public Health 77, 34 (2019). https://archpublichealth.biomedcentral.com/articles/10.1186/s13690-019-0355-z
- ³⁷ Martini, 1997. Researchers call for further studies after identifying a possible link between aspartame and brain tumors. https://www.sott.net/article/149206-Dr-John-Olney-on-Brain-tumors-and-aspartame
- ³⁸ Mandrioli et al, 2016. Relationship between Research Outcomes and Risk of Bias, Study Sponsorship, and Author Financial Conflicts of Interest in Reviews of the Effects of Artificially Sweetened Beverages on Weight Outcomes: A Systematic Review of Reviews. PLoS One. 2016 Sep 8;11(9):e0162198. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5015869/
- ³⁹ Azad et al, 2017. Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ. 2017 Jul 17;189(28):E929-E939. https://pmc.ncbi.nlm.nih.gov/articles/PMC5515645/
- ⁴⁰ Soffritti et al, 2005. First Experimental Demonstration of the Multipotential Carcinogenic Effects of Aspartame Administered in the Feed to Sprague-Dawley Rats. Environmental Health Perspectives Volume 114, Issue 3. Pages 379 - 385. https://ehp.niehs.nih.gov/doi/abs/10.1289/ehp.8711
- ⁴¹ Belpoggi et al, 2006. Results of Long-Term Carcinogenicity Bioassay on Sprague-Dawley Rats Exposed to Aspartame Administered in Feed. Annals of the New york Academy of Sciences. https://nyaspubs.onlinelibrary.wiley.com/doi/abs/10.1196/annals.1371.080
- ⁴² Soffritti et al, 2007. Life-Span Exposure to Low Doses of Aspartame Beginning during Prenatal Life Increases Cancer Effects in Rats. Environmental Health Perspectives, Volume 115, Issue 9, Pages 1293 - 129. https://ehp.niehs.nih.gov/doi/full/10.1289/ehp.10271
- ⁴³ Huff, 2006. Chemicals Studied and Evaluated in Long-Term Carcinogenesis Bioassays by Both the Ramazzini Foundation and the National Toxicology Program. Annuals of the New York Academy of Sciences, Volume 982, Issue 1. https://nyaspubs.onlinelibrary.wiley.com/doi/10.1111/j.1749-6632.2002.tb04935.x
- ⁴⁴ The Organisation for Economic Co-operation and Development, 2018. OECD 451. OECD Guideline for the testing of Chemicals: Carcinogenicity Studies. https://www.oecd.org/content/dam/oecd/en/publications/reports/2018/06/test-no-451-carcinogenicity-studies_g1gh2955/9789264071186-en.pdf
- ⁴⁵ European Food Safety Authority, 2006. EFSA assesses new aspartame study and reconfirms its safety. https://www.efsa.europa.eu/en/news/efsa-assesses-new-aspartame-study-and-reconfirms-its-safety
- ⁴⁶ Soffritti et al, 2014. The Carcinogenic Effects of Aspartame: The Urgent Need for Regulatory Re-Evaluation. American Jounal of Industral Medicine 57:383–397 (2014). https://www.ramazzini.org/wp-content/uploads/2009/02/The-carcinogenic-effects-of-aspartame_The-urgent-need-for-regulatory-re-evaluation-2014.pdf
- ⁴⁷ Huff et al, 2016. The limits of two-year bioassay exposure regimens for identifying chemical carcinogens. Environ Health Perspect. 2008 Nov;116(11):1439-42. https://pubmed.ncbi.nlm.nih.gov/19057693/
- ⁴⁸ Landrigan and Straif, 2021. Aspartame and cancer – new evidence for causation. Environ Health 20, 42 (2021). https://ehjournal.biomedcentral.com/articles/10.1186/s12940-021-00725-y
- ⁴⁹ Gnudi et al, 2023. Hemolymphoreticular Neoplasias from the Ramazzini Institute Long-term Mice and Rat Studies on Aspartame. Annals of Global Health, 89(1), p. 43. https://annalsofglobalhealth.org/articles/10.5334/aogh.4163
- ⁵⁰ International Agency for Research on Cancer, 2024. Aspartame, Methyleugenol, and Isoeugenol IARC Monographs on the Identification of Carcinogenic Hazards to Humans Volume 134. https://publications.iarc.who.int/627
- ⁵¹ Magnuson et al, 2007. Aspartame: a safety evaluation based on current use levels, regulations, and toxicological and epidemiological studies. Crit Rev Toxicol. 2007;37(8):629-727. https://pubmed.ncbi.nlm.nih.gov/17828671/
- ⁵² Aspartame.org, 2006. Comprehensive Review of Ramazzini Study Demonstrates No Scientific Evidence of Aspartame and Cancer Link. https://aspartame.org/comprehensive-review-ramazzini-study-demonstrates-no-scientific-evidence-aspartame-cancer-link/
- ⁵³ Soffritti, 2008. Carcinogenicity of Aspartame: Soffritti Responds. Environmental Health Perspectives, Volume 116, Issue 6, Page A240. https://ehp.niehs.nih.gov/doi/full/10.1289/ehp.10881R
- ⁵⁴ Collegium Ramazzini, 2011. Confirmation of the Experimental Carcinogenicity of Aspartame. https://collegiumramazzini.org/news/detail/211
- ⁵⁵ Center for Science in the Public Interest, 2013. Evaluation of Ramazzini Institute Aspartame Studies – and EFSA’s Assessment. Lisa Y. Lefferts, MSPH Senior Scientist. https://www.efsa.europa.eu/sites/default/files/event/documentset/130409-p06.pdf
- ⁵⁶ Foodwatch, 2025. Clean Washing Aspartame. Why aspartame needs to be banned, based on independent science. https://www.foodwatch.org/fileadmin/-INT/additives/2025-02-04_foodwatch-report-aspartame.pdf
- ⁵⁷ Réseau Environnement Santé, 2013. Analyse du rapport EFSA “Draft Opinion on the Re-Evaluation of Aspartame (E 951) as a Food Additive” https://reseau-environnement-sante.fr/wp-content/uploads/2016/01/AnalyseRapport-EFSAJanv2013-VF.pdf
- ⁵⁸ Marie-Monique Robin, 2011. Extract of the movie « Our Daily Poison » - https://www.youtube.com/watch?v=3CBXDU4cKNE
- ⁵⁹ Corporate Europe Observatory, 2011. Exposed: conflicts of interest among EFSA’s experts on food additives. https://corporateeurope.org/sites/default/files/publications/efsa_ans_panel.pdf
- ⁶⁰ U.S. Right to Know, 2023. International Life Sciences Institute (ILSI) is a food industry lobby group. September 16, 2023. https://usrtk.org/pesticides/ilsi-is-a-food-industry-lobby-group/
- ⁶¹ European Parliament, 2014. REPORT on discharge in respect of the implementation of the budget of the European Food Safety Authority for the financial year 2012. A7-0219/2014. https://www.europarl.europa.eu/doceo/document/A-7-2014-0219_EN.html?redirect
- ⁶² Corporate Europe Observatory, 2020. Conflicts of interest scandals at EFSA: A non-exhaustive chronology of recent events. https://corporateeurope.org/en/food-and-agriculture/efsa/chronology
- ⁶³ European Food Safety Authority, 2014. Decision of the Executive Director on Declarations of Interest. EFSA/LRA/DEC/02/2014. https://www.efsa.europa.eu/sites/default/files/corporate_publications/files/independencerules2014.pdf
- ⁶⁴ European Food Safety Authority, 2015. Increasing robustness, transparency and openness of scientific assessments. https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2015.e13031
- ⁶⁵ Regulation (EU) 2019/1381 of the European Parliament and of the Council - https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2019.231.01.0001.01.ENG&toc=OJ:L:2019:231:TOC
- ⁶⁶ Soffritti et al, 2014. The Carcinogenic Effects of Aspartame: The Urgent Need for Regulatory Re-Evaluation. American Journal of Industrial Medicine. https://www.ramazzini.org/wp-content/uploads/2009/02/The-carcinogenic-effects-of-aspartame_The-urgent-need-for-regulatory-re-evaluation-2014.pdf
- ⁶⁷ Doueihy et al, 2025. Aspartame and Human Health: A Mini-Review of Carcinogenic and Systemic Effects. J Xenobiot. 2025 Jul 7;15(4):114. https://pmc.ncbi.nlm.nih.gov/articles/PMC12286081/#notes2
- ⁶⁸ Le Point, 2011. Aspartame, un scandale sanitaire en cours. https://www.lepoint.fr/sante/aspartame-un-scandale-sanitaire-en-cours-15-09-2011-1373463_40.php
- ⁶⁹ International Agency for Research on Cancer, 2024. IARC Monographs Volume 134: Aspartame, methyleugenol, and isoeugenol. 13 September 2024. https://www.iarc.who.int/news-events/iarc-monographs-volume-134-aspartame-methyleugenol-and-isoeugenol/
- ⁷⁰ International Agency for Research on Cancer, 2024. Aspartame, methyleugenol, and isoeugenol. IARC Monographs on the Identification of Carcinogenic Hazards to Humans, No. 134. https://www.ncbi.nlm.nih.gov/books/NBK609291/
- ⁷¹ Debras et al, 2022. Artificial sweeteners and cancer risk: Results from the NutriNet-Santé population-based cohort study. PLoS Med. 2022 Mar 24;19(3):e1003950. https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1003950
- ⁷² Schernhammer et al, 2012. Consumption of artificial sweetener- and sugar-containing soda and risk of lymphoma and leukemia in men and women. Am J Clin Nutr. 2012 Dec;96(6):1419-28. https://www.sciencedirect.com/science/article/pii/S0002916523029209?via%3Dihub
- ⁷³ Landrigan et al, 2025. Irregularities in the IARC Working Group Evaluation of Ramazzini Institute Aspartame Studies. Ann Glob Health. 2025 Jun 6;91(1):28. https://pmc.ncbi.nlm.nih.gov/articles/PMC12143253/
- ⁷⁴ World Health Organization, 2024. Diabetes. https://www.who.int/news-room/fact-sheets/detail/diabetes
- ⁷⁵ World Health Organization, 2022. Health effects of the use of non-sugar sweeteners: a systematic review and meta-analysis. https://www.who.int/publications/i/item/9789240046429
- ⁷⁶ Fagherazzi et al, 2013. Consumption of artificially and sugar-sweetened beverages and incident type 2 diabetes in the Etude Epidemiologique aupres des femmes de la Mutuelle Generale de l'Education Nationale-European Prospective Investigation into Cancer and Nutrition cohort. Am J Clin Nutr. 2013 Mar;97(3):517-23. https://www.sciencedirect.com/science/article/pii/S0002916523054412?via%3Dihub
- ⁷⁷ Nettleton et al, 2009. Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care. 2009 Apr;32(4):688-94. https://pmc.ncbi.nlm.nih.gov/articles/PMC2660468/
- ⁷⁸ Sakurai et al, 2014. Sugar-sweetened beverage and diet soda consumption and the 7-year risk for type 2 diabetes mellitus in middle-aged Japanese men. Eur J Nutr. 2014 Feb;53(1):251-8. doi: 10.1007/s00394-013-0523-9. https://pubmed.ncbi.nlm.nih.gov/23575771/
- ⁷⁹ Gul et al, 2017. Inhibition of the gut enzyme intestinal alkaline phosphatase may explain how aspartame promotes glucose intolerance and obesity in mice. Appl Physiol Nutr Metab. 2017 Jan;42(1):77-83. https://cdnsciencepub.com/doi/full/10.1139/apnm-2016-0346
- ⁸⁰ Fowler et al, 2012. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity (Silver Spring). 2008 Aug;16(8):1894-900. https://onlinelibrary.wiley.com/doi/full/10.1038/oby.2008.284
- ⁸¹ Anton et al, 2010. Effects of stevia, aspartame, and sucrose on food intake, satiety, and postprandial glucose and insulin levels. Appetite. 2010 Aug;55(1):37-43. https://www.sciencedirect.com/science/article/abs/pii/S0195666310000826
- ⁸² Suez et al, 2022. Personalized microbiome-driven effects of non-nutritive sweeteners on human glucose tolerance. Cell. 2022 Sep 1;185(18):3307-3328.e19. https://www.cell.com/cell/fulltext/S0092-8674(22)00919-9
- ⁸³ Debras et al, 2023. Artificial Sweeteners and Risk of Type 2 Diabetes in the Prospective NutriNet-Santé Cohort. Diabetes Care. 2023 Sep 1;46(9):1681-1690. https://pmc.ncbi.nlm.nih.gov/articles/PMC10465821/
- ⁸⁴ Malik and Hu, 2012. Sweeteners and Risk of Obesity and Type 2 Diabetes: The Role of Sugar-Sweetened Beverages. Curr Diab Rep. 2012 Jan 31. https://link.springer.com/article/10.1007/s11892-012-0259-6
- ⁸⁵ World Health Organization, 2025. Cardiovascular diseases (CVDs). https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
- ⁸⁶ Debras et al, 2022. Artificial sweeteners and risk of cardiovascular diseases: results from the prospective NutriNet-Santé cohort. BMJ. 2022 Sep 7;378:e071204. https://www.bmj.com/content/378/bmj-2022-071204.full
- ⁸⁷ World Health Organization, 2023. Use of non-sugar sweeteners. WHO guideline. https://iris.who.int/bitstream/handle/10665/367660/9789240073616-eng.pdf?sequence=1
- ⁸⁸ Zhang et al, 2025. Aspartame and ischemic stroke: unraveling the molecular link through network toxicology and molecular docking analysis. Sci Rep. 2025 Jul 4;15(1):23871. https://www.nature.com/articles/s41598-025-08898-z
- ⁸⁹ Valdes et al, 2018. Role of the gut microbiota in nutrition and health. BMJ. 2018 Jun 13;361:k2179. https://www.bmj.com/content/361/bmj.k2179
- ⁹⁰ Qin et al, 2012. A Metagenome-Wide Association Study of the Gut Microbiome and Metabolic Syndrome. Front Microbiol. 2021 Jul 16;12:682721. https://www.nature.com/articles/nature11450
- ⁹¹ Huybrechts et al, 2021. The Human Microbiome in Relation to Cancer Risk: A Systematic Review of Epidemiologic Studies. Cancer Epidemiol Biomarkers Prev. 2020 Oct;29(10):1856-1868. https://pmc.ncbi.nlm.nih.gov/articles/PMC7541789/#S15
- ⁹² Tang et al, 2013. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013 Apr 25;368(17):1575-84. https://www.nejm.org/doi/full/10.1056/NEJMoa1109400
- ⁹³ Baothman et al, 2016. The role of Gut Microbiota in the development of obesity and Diabetes. Lipids Health Dis. 2016 Jun 18;15:108. https://link.springer.com/article/10.1186/s12944-016-0278-4
- ⁹⁴ Suez et al, 2014. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014 Oct 9;514(7521):181-6. https://pubmed.ncbi.nlm.nih.gov/25231862/
- ⁹⁵ Palmnäs et al, 2014. Low-dose aspartame consumption differentially affects gut microbiota-host metabolic interactions in the diet-induced obese rat. PLoS One. 2014 Oct 14;9(10):e109841. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0109841
- ⁹⁶ Shil et al, 2020. Artificial Sweeteners Disrupt Tight Junctions and Barrier Function in the Intestinal Epithelium through Activation of the Sweet Taste Receptor, T1R3. Nutrients. 2020 Jun 22;12(6):1862. https://pmc.ncbi.nlm.nih.gov/articles/PMC7353258/
- ⁹⁷ Pepino, 2016. Metabolic effects of non-nutritive sweeteners. Physiol Behav. 2015 Dec 1;152(Pt B):450-5. https://pmc.ncbi.nlm.nih.gov/articles/PMC4661066/
- ⁹⁸ Rother et al, 2018. How Non-nutritive Sweeteners Influence Hormones and Health. Trends Endocrinol Metab. 2018 Jul;29(7):455-467. https://pubmed.ncbi.nlm.nih.gov/29859661/
- ⁹⁹ Angelin et al, 2024. Artificial sweeteners and their implications in diabetes: a review. Front Nutr. 2024 Jun 25;11:1411560. https://pmc.ncbi.nlm.nih.gov/articles/PMC11233937/#B5
- ¹⁰⁰ Olney and Ho, 1970. Brain damage in infant mice following oral intake of glutamate, aspartate or cysteine. Nature. 1970 Aug 8;227(5258):609-11. https://www.nature.com/articles/227609b0
- ¹⁰¹ Olney, 1979. Excitotoxic Amino Acids: Research Applications and Safety Implications. https://www.ajinomoto.com.my/sites/default/files/paragraph/side-by-side/files/excitotoxic-amino-acids.pdf
- ¹⁰² Humphries et al, 2008. Direct and indirect cellular effects of aspartame on the brain. Eur J Clin Nutr. 2008 Apr;62(4):451-62. https://www.nature.com/articles/1602866
- ¹⁰³ Choudhar and Lee, 2018. The debate over neurotransmitter interaction in aspartame usage. J Clin Neurosci. 2018 Oct;56:7-15. https://www.sciencedirect.com/science/article/abs/pii/S0967586818305770
- ¹⁰⁴ Ashok et al, 2015. Neurobehavioral changes and activation of neurodegenerative apoptosis on long-term consumption of aspartame in the rat brain. Journal of Nutrition & Intermediary Metabolism, Vol. 2, Issues 3-4, December 2015, Pages 76-85. https://www.sciencedirect.com/science/article/pii/S2352385915300025
- ¹⁰⁵ Jones et al, 2023. Learning and memory deficits produced by aspartame are heritable via the paternal lineage. Sci Rep. 2023 Aug 31;13(1):14326. https://www.nature.com/articles/s41598-023-41213-2
- ¹⁰⁶ Bai et al, 2024. Non-nutritive Sweetener Aspartame Disrupts Circadian Behavior and Causes Memory Impairment in Mice. J Agric Food Chem. 2024 Oct 23;72(42):23478-23492. https://pubmed.ncbi.nlm.nih.gov/39382230/
- ¹⁰⁷ European Food Safety Authority, 2025. ADI. https://www.efsa.europa.eu/en/glossary/adi
- ¹⁰⁸ Joint FAO/WHO Expert Committee on Food Additives, 1980. Evaluation of certain food additive. Twenty-fourth Report of the Joint FAO/WHO Expert Committee on Food Additives. https://iris.who.int/bitstream/handle/10665/41410/WHO_TRS_653.pdf?sequence=1
- ¹⁰⁹ Food and Drug Administration, 2023. Timeline of Selected FDA Activities and Significant Events Addressing Aspartame. https://www.fda.gov/food/food-additives-petitions/timeline-selected-fda-activities-and-significant-events-addressing-aspartame
- ¹¹⁰ Gordon 1987. News Story. https://www.sussex.ac.uk/webteam/gateway/file.php?name=document-24-greg-gordon-upi-12oct1987.pdf&site=25
- ¹¹¹ Food and Drug Administration, 2023. Timeline of Selected FDA Activities and Significant Events Addressing Aspartame. https://www.fda.gov/food/food-additives-petitions/timeline-selected-fda-activities-and-significant-events-addressing-aspartame
- ¹¹² Corporate Europe Observatory, 2012. Conflits indigestes ! Une décennie d’influence industrielle à l'Autorité Européenne de Sécurité Alimentaire (EFSA). http://www.adequations.org/IMG/pdf/conflits_indigestes.pdf
- ¹¹³ European Food Safety Authority, 2013. Scientific Opinion on Aspartame. https://www.efsa.europa.eu/sites/default/files/corporate_publications/files/factsheetaspartame.pdf
- ¹¹⁴ World Health Organization, 2023. Evaluations of the Joint FAO/WHO Expert Committee on FDood Additives (JECFA). Aspartame. https://apps.who.int/food-additives-contaminants-jecfa-database/Home/Chemical/62
- ¹¹⁵ World Health Organization, 2023. Aspartame hazard and risk assessment results released. https://www.who.int/news/item/14-07-2023-aspartame-hazard-and-risk-assessment-results-released
- ¹¹⁶ French Agency for Food, Environmental and Occupational Health & Safety, 2021. The European Transparency Regulation: a new framework for risk assessment and food safety. https://www.anses.fr/en/content/european-transparency-regulation-new-framework-risk-assessment-and-food-safety
- ¹¹⁷ Joint FAO/WHO Expert Committee on Food Additives, 2023. Evaluation of certain food additives? Ninety-sixth report of the Joint FAO/WHO Expert Committee on Food Additives. https://iris.who.int/bitstream/handle/10665/376279/9789240083059-eng.pdf?sequence=1
- ¹¹⁸ Joint FAO/WHO Expert Committee on Food Additives, 2024. Comité du codex sur les additifs alimentaires. Questions d’intérêt découlant de la FAO et l’OMS et des 96ème et 97ème réunions du Comité mixte FAO/OMS d’Experts des Additifs Alimentaires (JECFA) respectivement. https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FMeetings%252FCX-711-54%252FWorking%2Bdocuments%252Ffa54_03f.pdf
- ¹¹⁹ Foodwatch, 2025. Clean Washing Aspartame. Why aspartame needs to be banned, based on
- independent science. https://www.foodwatch.org/fileadmin/-INT/additives/2025-02-04_foodwatch-report-aspartame.pdf
- ¹²⁰ U.S. Right to Know, 2023. Did a Coca-Cola front group sway a WHO review of Aspartame ? Gary Ruskin, July 19, 2023. https://usrtk.org/sweeteners/coca-cola-front-group-who-review-of-aspartame/
- ¹²¹ Walton et al, 1999. The application of in vitro data in the derivation of the acceptable daily intake of food additives. Food Chem Toxicol. 1999 Dec;37(12):1175-97. https://pubmed.ncbi.nlm.nih.gov/10654594/
- ¹²² Galli et al, 2008. Is the acceptable daily intake as presently used an axiom or a dogma? Toxicol Lett. 2008 Aug 15;180(2):93-9. https://pubmed.ncbi.nlm.nih.gov/18588960/
- ¹²³ Phillips et al, 2024. Improving the integration of epidemiological data into human health risk assessment: What risk assessors told us they want. Glob Epidemiol. 2024 Sep 28;8:100167. https://www.sciencedirect.com/science/article/pii/S2590113324000336
- ¹²⁴ Debras et al, 2022. Artificial sweeteners and cancer risk: Results from the NutriNet-Santé population-based cohort study. PLoS Med 19(3): e1003950. https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1003950
- ¹²⁵ Debras et al, 2022. Artificial sweeteners and risk of cardiovascular diseases: results from the prospective NutriNet-Santé cohort BMJ 2022; 378 :e071204. https://www.bmj.com/content/378/bmj-2022-071204.full
- ¹²⁶ Debras et al, 2023. Artificial Sweeteners and Risk of Type 2 Diabetes in the Prospective NutriNet-Santé Cohort. Diabetes Care. 2023 Sep 1;46(9):1681-1690. https://pmc.ncbi.nlm.nih.gov/articles/PMC10465821/
- ¹²⁷ Chia et al, 2016. Chronic Low-Calorie Sweetener Use and Risk of Abdominal Obesity among Older Adults: A Cohort Study. PLoS One. 2016 Nov 23;11(11):e0167241. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0167241#sec021
- ¹²⁸ Azad et al, 2020. Nonnutritive sweetener consumption during pregnancy, adiposity, and adipocyte differentiation in offspring: evidence from humans, mice, and cells. Int J Obes (Lond). 2020 Oct;44(10):2137-2148. https://www.nature.com/articles/s41366-020-0575-x
- ¹²⁹ Food and Agriculture Organization, 2025. State of Research on the Interactions between Food Additives, the Gut Microbiome and the Host. A Food Safety Perspective. Food Safety and Quality Series. https://openknowledge.fao.org/server/api/core/bitstreams/54e3c4a1-e95b-4365-b9ae-f99a75436106/content
- ¹³⁰ French Agency for Food, Environmental and Occupational Health & Safety, 2015. Évaluation
- des bénéfices et des risques nutritionnels des édulcorants intenses. Avis de l’Anses, Rapport d’expertise collective, Janvier 2015. https://www.anses.fr/fr/system/files/NUT2011sa0161Ra.pdf
- ¹³¹ Engel et al, 2018. Effect of high milk and sugar-sweetened and non-caloric soft drink intake on insulin sensitivity after 6 months in overweight and obese adults: a randomized controlled trial. Eur J Clin Nutr. 2018 Mar;72(3):358-366. https://pubmed.ncbi.nlm.nih.gov/29235560/
- ¹³² Chia et al, 2016. Chronic Low-Calorie Sweetener Use and Risk of Abdominal Obesity among Older Adults: A Cohort Study. PLoS One. 2016 Nov 23;11(11):e0167241. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0167241#sec021
- ¹³³ French Agency for Food, Environmental and Occupational Health & Safety, 2015. Évaluation
- des bénéfices et des risques nutritionnels des édulcorants intenses. Avis de l’Anses, Rapport d’expertise collective, Janvier 2015. https://www.anses.fr/fr/system/files/NUT2011sa0161Ra.pdf
- ¹³⁴ Pepino, 2016. Metabolic effects of non-nutritive sweeteners. Physiol Behav. 2015 Dec 1;152(Pt B):450-5. doi: 10.1016/j.physbeh.2015.06.024. Epub 2015 Jun 19. https://pmc.ncbi.nlm.nih.gov/articles/PMC4661066/
- ¹³⁵ Rother et al, 2018. How Non-nutritive Sweeteners Influence Hormones and Health. Trends Endocrinol Metab. 2018 Jul;29(7):455-467. https://www.sciencedirect.com/science/article/abs/pii/S1043276018300936
- ¹³⁶ Regulation No 178/2002 of the European Parliament and of the Council of 28 January 2002. https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:02002R0178-20240701





Thank you Yuka for all this information I really needed it very necessary to know what we are eating and what we are getting in our food
I really appreciate the way you’ve highlighted the history of political and corporate influence in food regulation. Those concerns are real, and the aspartame story is a perfect example of why transparency and independence in science matter. The revolving door between regulators and industry deserves more attention, so thank you for shining a light on it.
That said, when it comes to aspartame itself, the totality of evidence is a bit more nuanced. For example:
• The Ramazzini Institute rat studies that raised cancer concerns were later judged unreliable by both EFSA and FDA because of major methodological flaws.
• Large human studies, such as the NIH-AARP cohort (>500,000 people) and the National Cancer Institute’s 2006 study, found no consistent link between aspartame and cancer.
• EFSA’s 2013 re-evaluation reviewed more than 600 studies and concluded that aspartame is safe at current consumption levels, except for people with PKU.
That doesn’t mean the debate is closed — the WHO/IARC’s 2023 “possibly carcinogenic” classification shows that weak signals in observational data (which does not prove cause and effect necessarily) but it still need exploring. But at present, the weight of evidence suggests aspartame is safe for the general population at typical intake.
So while political capture is a valid concern in food policy overall, the science on aspartame looks less alarming than some headlines imply. Perhaps a follow-up could weigh those contrasting findings side by side — it would make your already strong piece even more robust.
Hi Tim, I was sharing some information with a friend about aspartame and came across this article and your response. I would encourage you to look at other scientific articles about the effects of aspartame. Without going into all the details, I started researching aspartame when I had an episode of memory loss and confusion (I didn’t know why I was on the bus or where I was headed – it was the same bus I had taken for years to work). When I sat down to my desk that morning, I didn’t know how to print out a document from my computer – I had been an administrative assistant for 15 years and printed pages daily.
I had had multiple tests through a neuropsychiatrist to check for MS, Dementia, and ALS – so I had run all the test with no answers. Then the doctor did a manual-type memory test, and he said my results were all over the place and he couldn’t diagnose me.
I was telling a woman at work, and she shared that she had one similar experience herself. She ate all whole foods, but she said she allowed herself one treat – Diet Coke. She said that after she quit drinking Diet Coke, she never had another episode. I immediately quick drinking Diet Coke, and I also quit chewing sugar-free gum, eating sugar-free candy, cough drops, or anything that had aspartame in it. There is a lot of diabetes in my family, so I was trying to be super careful about sugar intake.
Two weeks after stopping all aspartame, I had a follow-up with my doctor. When I told him about the aspartame, he ran the same memory tests that he had run two weeks prior. He was floored at the difference of the tests, and he vowed to look into studies on aspartame.
So we are only two people, but I have talked to other people and read lots of research, and I can tell you there are a lot of people who have had the same experience after using aspartame. The first article I read about aspartame was written by a doctor who quit the FDA because he couldn’t get anyone to look at his research because there was too much money to be made. Here we are 25 years later still trying to hold the FDA accountable.
Since I quit using aspartame, I’ve never had another episode.
Wow! My mind is blown. This is terrifying. Thank you Yuka.
Thank you.
Twice after consuming aspartame, I was suddenly unable to read. I could see and name the letters but I had no idea what the words were. I recovered over a period of hours. I have since learned from Oliver Sacks that this symptom can be a form of migraine. I refuse to consume aspartame again.
I’ve always said approval from the FDA, CDC, etc. means nothing to me. As a matter of fact, their approval only serves to make me stay away from whatever they endorse.
Thank you Yuka for all that you do to make us aware of the dangerous ingredients we are deliberately allowed to consume for profits.
Aspartame should be made more visible on labelling rather than in the “small print” ingredients list – perhaps an added colour to the traffic light system would aid consumers when buying food/drink
We rely on honesty and transparency when making decisions about food purchasing. The behaviour of the food standards agencies that enable and supports the misrepresentation of data in the food industry is frankly shocking.
This needs to change NOW to allow us to make informed choices in our purchasing of food.
My partner and I are both sensitive to Aspartame – anything containing it tastes bitter to us. We have been avoiding it for years plus many other additives. We have a simple rule – if we can’t have a packet of it in the store cupboard/fridge/freezer, we don’t want it. This always involves careful scrutiny of ingredients in sauces etc, but this is where Yuka is so useful.
I did see an article this week about the rise in non-smoking related lung cancers amongst young fit people…….the report cited pollution as a possible cause when they go out for their daily exercise, but in the light of this report, I now wonder if aspartame might feature anywhere in this trend. More research needed there maybe?
I have noticed that aspartame gives me severe headache. First noticed when drinking Pepsi max when it first came out. I avoid aspartame aculsafame etc where possible. These sweeteners are in nearly all brands of cordials even the standard non diet ones. It infuriates me that these companies put them in in addition to sugar. Why do they do this?
Apparently it was to avoid paying the sugar tax, when it came in in some countries. Not 100% sure though.
Aspartame safety: Regulatory bodies like the FDA, EFSA (European Food Safety Authority), and WHO have all reviewed aspartame. They set an acceptable daily intake (ADI), about 40 mg per kg of body weight (EFSA). For someone weighing 70 kg (154 lbs), that’s around 2,800 mg per day, which equals 15–20 cans of diet soda depending on the brand. That’s a lot.
Cancer concerns: In 2023, the WHO’s cancer research agency (IARC) classified aspartame as “possibly carcinogenic” (Group 2B). That sounds scary, but it’s the same category as pickled vegetables, aloe vera, and being a carpenter. The classification means there’s limited evidence, not that it’s proven dangerous at normal intake levels.
Regulators’ stance: WHO’s food safety branch (JECFA) and EFSA both said there’s no need to change intake guidelines, and that normal consumption is safe. The risk would only appear if someone was drinking extreme, unrealistic amounts daily
For me the WHO is not an agency I trust.
Major safety reviews disagree on hazard vs risk: IARC (the cancer-classification body) classified aspartame as “possibly carcinogenic” (Group 2B) in July 2023 on the basis of limited human evidence and some animal signals, while risk assessors such as JECFA/FAO-WHO, EFSA, and the U.S. FDA have concluded that current exposures below the Acceptable Daily Intake (ADI) do not pose a public-health concern.
U.S. Food and Drug Administration
IARC
World Health Organization
The disagreement is largely methodological: IARC answers “can this substance cause cancer (hazard)?” using limited human/animal/ mechanistic evidence. JECFA/EFSA/FDA answer “at typical dietary exposures, is this a public health risk (risk)?” using exposure estimates and toxicology. Those are different questions and can produce different conclusions.
IARC
If you want to avoid WHO as a source, you can still evaluate the literature by (1) reading the IARC monograph (hazard), (2) reading JECFA/EFSA/FDA risk assessments (exposure + safety margins), (3) looking at the primary human epidemiology and the major animal studies (esp. Ramazzini Institute work and critiques), and (4) checking declarations of funding/conflicts for authors.
This is text book scare mongering. Here is the full list of organisations which have approved Aspartame for human consumption. They have no vested interest – indeed most have social medical systems which would incur huge costs if this ingredient was harmful.
United States,U.S. Food and Drug Administration,FDA
European Union,European Food Safety Authority; European Commission,EFSA; EC
United Kingdom,Food Standards Agency; Food Standards Scotland,FSA; FSS
Canada,Health Canada,HC
Australia & New Zealand,Food Standards Australia New Zealand,FSANZ
Japan,Ministry of Health Labour and Welfare; Food Safety Commission of Japan,MHLW; FSCJ
China,National Health Commission; State Administration for Market Regulation,NHC; SAMR
India,Food Safety and Standards Authority of India,FSSAI
Brazil,National Health Surveillance Agency,ANVISA
Mexico,Federal Commission for Protection against Sanitary Risk,COFEPRIS
South Korea,Ministry of Food and Drug Safety,MFDS
Singapore,Singapore Food Agency,SFA
Malaysia,Ministry of Health (Food Safety and Quality Division),MOH (FSQD)
Indonesia,National Agency of Drug and Food Control,BPOM
Philippines,Food and Drug Administration (Food and Drug Regulation),FDA Philippines
Thailand,Thai Food and Drug Administration,Thai FDA
Vietnam,Vietnam Food Administration; Ministry of Health,VFA; MOH
Russia, Rospotrebnadzor (Consumer Protection and Human Wellbeing); Eurasian Economic Commission,Rospotrebnadzor; EEC
Eurasian Economic Union,Eurasian Economic Commission (Technical Regulation),EEC
Turkey,Ministry of Agriculture and Forestry (General Directorate of Food and Control),MAF (GDFC)
Saudi Arabia,Saudi Food and Drug Authority,SFDA
United Arab Emirates,Ministry of Climate Change and Environment; Emirates Authority for Standardization and Metrology,MOCCAE; ESMA
Qatar,Ministry of Public Health (Food Safety Department),MOPH
Kuwait,Public Authority for Food and Nutrition,KPAFN
Oman,Food Safety and Quality Center (Ministry of Agriculture Fisheries and Water Resources),FSQC
Bahrain,National Health Regulatory Authority; Ministry of Health,NHRA; MOH
South Africa,National Department of Health (Food Control),NDoH
Nigeria,National Agency for Food and Drug Administration and Control,NAFDAC
Egypt,National Food Safety Authority,NFSA
Israel,Ministry of Health – National Food Service,MOH
Argentina,National Administration of Drugs Foods and Medical Devices; National Food Commission,ANMAT; CONAL
Chile,Ministry of Health (Food and Nutrition Dept.),MINSAL
Colombia,National Institute for Food and Drug Surveillance,INVIMA
Peru,General Directorate of Environmental Health and Food Safety,DIGESA
Switzerland,Federal Food Safety and Veterinary Office,FSVO (BLV/OSAV)
Norway,Norwegian Food Safety Authority,Mattilsynet
Iceland,Icelandic Food and Veterinary Authority,MAST
Hong Kong,Center for Food Safety (Food and Environmental Hygiene Dept.),CFS
Taiwan,Taiwan Food and Drug Administration,TFDA
Pakistan,Pakistan Standards and Quality Control Authority; Ministry of National Health Services,PSQCA; MoNHS
Bangladesh,Bangladesh Food Safety Authority,BFSA
Sri Lanka,Food Control Administration Unit (Ministry of Health),MOH
New Zealand,Food Standards Australia New Zealand (shared system),FSANZ
As it says in the article, these companies have relied on the dodgy data from the 80’s in the US instead of doing their own proper research.
Rob. You have replied to every comment. Stop this obsession lol. It is safe, have a diet coke and calm down mate.
This is not text book scaremongering at all.
Just because these organisations have apparently approved of aspartame…..doesn’t mean anything at all!!!!
It just goes to prove they’re either ignorant, don’t care or are part of the problem!!!
***Keep away from any food product that are artificial…..also check the ingredients on labels***
100s of countries, 1000s of scientists and healthcare professionals, the world’s most respected food standards agencies. But Sandra knows best…
top brave independent article
I wonder what’s included in the ‘Others’ section of food categories (4.3%)? What about toothpastes and medications, many of which contain sweeteners. I checked and Gaviscon Advance tablets contain Aspartame and there are various sweeteners in my toothpastes. The trouble is that most of these tend to be used or must be used on a daily basis, so you still absorb sweeteners even though you avoid all those nasty soft drinks. How do the amounts in toothpastes and medications compare with the amounts in other food groups? Are they really necessary?
If it’s unavoidable then go ahead, but drinking 3 cans of diet coke a day isn’t unavoidable. I’d rather have actual sugar than some man-made chemical invented in some lab.
I rely on the Yuka app to scan and review all the ingredients in products. It helps me find toothpaste without harmful artificial sweeteners.
Additionally, I’ve discovered that instead of using products like Gavison for heartburn relief, slicing a piece of ginger root and adding it to my water not only enhances the flavor but also effectively reduces heartburn on most days. It also helps with bloating and belching. It’s all about finding the natural food items that work for you.
I have started using YUKA for my day to day stuff. It really increases the awareness about what we are eating. After that choices are dependent individuals. But me personally keeping it simple. because anything and everything you scan will have chemicals to make it taste nicer, increases shelf life, colouring and texturing agents. But if one eat fruits and vegetables, rice and home made bread. you can reduce these intake of chemicals to extra ordinary level where your body can cope with it. Especially if one stop drinking fizzy drinks and stop eating snacks and chocolates. it will help a lot
Thank you
https://www.youtube.com/watch?v=vf-Uvbaia1s
Your ‘research’ above is woefully inadequate to say the least, relying mostly on fear mongering about ‘Big Sweetener’. Correlation does not equal causation and everything is toxic even water if consumed in too high amounts.
How is it “inadequate”? It literally covers all bases, quoting both sides of the argument. Tells us how it was invented and the sneaky tactics they used to push through the legislation to allow it into our food and drink. Why would you trust the word of some food manufacturing fat cats who skewed the research to make it look safe? I’ll never touch the stuff again but you can chug it 24/7 if you want!
I have been more aware since joining Yuka, checking ingredients, and changing my choices. Plastics in everything, water too, nightmare…
I have found in my makeup/cosmetics that it’s not the brand, but the particular item. Mascara, for instance, L’Oreal, many scan harmful, but one L’Oreal mascara comes up excellent. Lo and behold, it is less expensive. I have found this with deodorant, shampoo, and eyeshadow.
As someone who has a bad allergy to sweeteners i hate seeing it everywhere. Sometimes at a show or our for a meal i cant get a single drink other than water and honestly its annoying .
But my views are that of someone who just wants to eat or drink with out fear of an allergic reaction
My only concern is that the authors here show associations, but not established or probable causal links.
Maybe the cited sources do include that, but the article does not.
I would check out the cited sources myself as the article would be much longer if all the data from the sources was included.
I drunk minutes bed now that sprite on the picture
Brilliant, thank you, I wonder how much of this stuff we’re all putting into our bodies, I do tend use the Yuka app but not aways, I need to take more care
I scan everything I possibly can before it goes into my shopping cart. It is now a habit. Mostly eating healthier by far these days. Let’s say I want dark chocolate, trail mix, or potato chips. I will scan and pick the healthiest of those scanned, or pick an alternative that Yuka offers if my store has it. I see this as healthier and less expensive. It does not go into my cart without a conscious decision.
Very interesting information which everyone should be made aware of. Let’s hope in time it reaches out to the Government to make these changes to stop including it in products. Well done Yuka! 👏 👏
Brilliant piece of journalism, thank you! Everyone needs to know this in order to make informed buying decisions. I will continue to tell friends about the Yuka app.
As ever in this ever increasing vulgar world, money means more than people’s life’s.
Great work, thanks for sharing. People need to be aware of this.
Exceptional work, well done.