
Introduction
Food dyes, preservatives, sweeteners… Additives are everywhere in American products. Their use is often poorly regulated, with a much looser regulatory framework than in Europe. As a result, many food additives are allowed without any quantity limits, and their evaluation is based on outdated or even controversial scientific data. Worse still, some additives that are banned in many countries remain authorized on the U.S. market.
How can such a discrepancy be explained? Why does the American system struggle to protect consumers?
Between the lack of rigor in the evaluation system, insufficiently independent regulations, and the influence of industry, here is our investigation into the flaws of this model.
The Ubiquity of Additives in American Products
American products contain significantly more additives than those sold in Europe. According to data from Yuka’s product database, which includes more than 3 million food products, processed foods in the U.S. contain an average of 3.1 additives per product, compared to 1.9 in France and Germany—63% more.1 The gap is also significant with other countries such as Canada (2.5 additives per product on average, 24% fewer) and even the UK (2.9 additives, 7% fewer).
A particularly striking example is store-bought bread: in the U.S., it contains an average of six additives per product, which is 36% more than the average for this category, which stands at 4.4 additives (based on an analysis of 16,372 products).
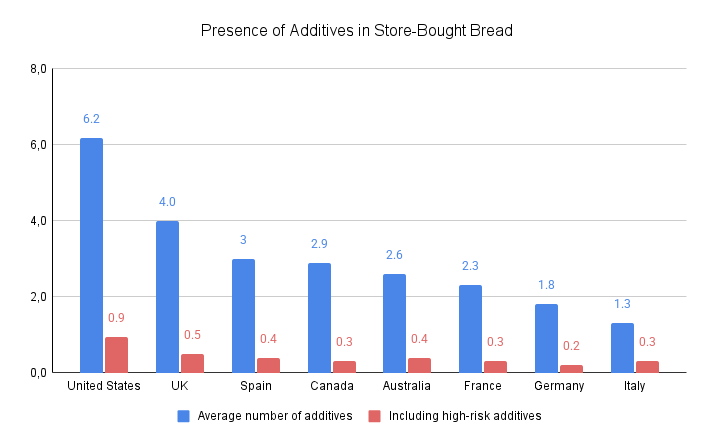
Beyond the sheer number of additives present in American products, their potential dangers are also concerning: several are banned in the European Union due to health risks. One such example is titanium dioxide, a white food dye widely used in candies, biscuits, and pastries. Since January 2022, it has been banned in food products in the European Union due to its potential carcinogenic2 and genotoxic effects (damage to cell DNA).3,4 Many other countries, including Turkey, Qatar, and Saudi Arabia, have followed suit.5 In the U.S., however, it remains legal and widely used.
Another alarming example is propylparaben, which is still used as a preservative in some baked goods in the U.S. In Europe, this additive has been banned since 20066 due to its harmful effects on reproduction, observed at the hormonal level and on male reproductive organs.7 These effects were detected at concentrations so low that the European Food Safety Authority (EFSA) deemed it impossible to establish a safe threshold. Propylparaben is also banned in China.8 However, despite these risks, it remains authorized in the U.S., where it is classified as a “Generally Recognized as Safe” (GRAS) substance.9 In 2023, California took the lead by deciding to ban it, with the measure set to take effect in January 2027.10
These prime examples actually pertain to only a limited number of additives. In reality, only twelve additives commonly used in the United States are banned in the European Union11. While these cases often receive media attention, they overshadow a deeper issue: the overall regulation of additive use, which is far more lenient in the U.S. Europe applies an approach based on the precautionary principle, with stricter—though still imperfect—conditions of use. In contrast, the American regulatory system, lacking sufficient safeguards, allows for a much more unrestricted use of food additives.
Sodium aluminum sulfate illustrates this problem well. This additive contains aluminum, which is suspected of affecting reproduction, fetal development, and contributing to diseases (Alzheimer’s, autism, and epilepsy)12-14 due to its accumulation in the body.15 Sodium aluminum sulfate is allowed on both sides of the Atlantic. However, its use is far more restricted in Europe, where it is limited to specific products such as candied cherries and liquid egg whites.16 In the U.S., however, it is permitted in any type of food and is widely used in everyday items such as bread, cakes, and tortillas, leading to significantly higher exposure for American consumers.17
Furthermore, the dosage of additives in the American market is also largely unregulated. As a result, there are few strict limits on the maximum concentration allowed for each additive. Among the 50 most common additives that present a certain level of danger according to Yuka18, 38% have no usage restrictions in the U.S., compared to only 14% in the European Union. In other words, the U.S. imposes nearly three times fewer restrictions than Europe.
Some additives are particularly concerning when consumed in high quantities. One example is Blue 1, which has been linked to hyperactivity and attention disorders in children and is suspected of toxicity to the immune system and blood.19,20 The EFSA has warned that its safety threshold could be exceeded in children.21 As a result, its use is restricted in the European Union to a limited number of products, with strict maximum concentrations. For instance, it is limited to 0.015% in ice cream and flavored yogurt, and 0.03% in candies—three product categories frequently consumed by children.15
In the U.S., however, this dye is permitted in all types of products without any maximum limit.22 A 2015 independent study23 found extremely high concentrations of Blue 1 in several products. Some cupcakes and candies like Skittles contained up to twice the European limit. It was also found in children’s cereals at a concentration of 0.13%. In Europe, however, this additive is banned in breakfast cereals, and the highest permitted concentration in the few allowed products is 0.05%—three times lower.
Even when limits do exist in the U.S., they are generally much higher than in Europe. For example, the maximum concentration of sodium nitrites allowed in the U.S. can be up to four times the latest limit set by the European Union for certain processed products, such as ham, sausages, and poultry-based foods.24 Nitrites are classified as probably carcinogenic due to their suspected link to colon and stomach cancers.25
But how can such a disparity be explained? Several structural and regulatory factors contribute to this situation, making the U.S. system less stringent and slower to react than the one in place in Europe.
A System Without Real Safeguards
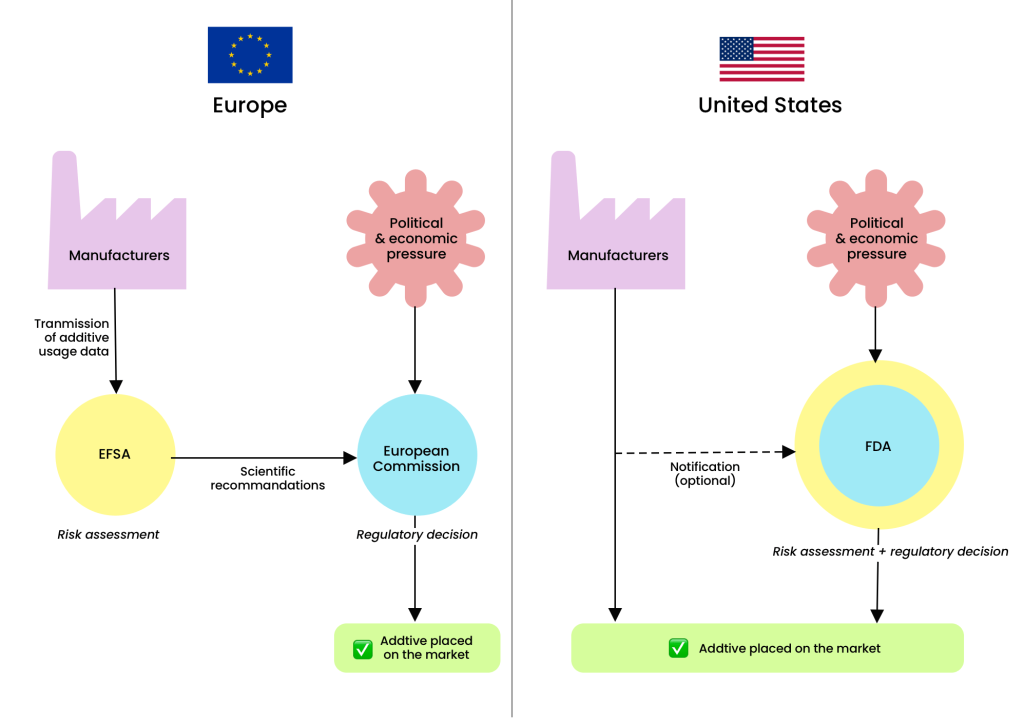
One of the major differences between the U.S. and European systems lies in the separation of responsibilities between public agencies. In the European Union, the evaluation and management of risks related to food additives are strictly divided: the EFSA assesses risks and issues recommendations, while the European Commission (EC) makes regulatory decisions. The EFSA, composed of independent experts, focuses exclusively on scientific evaluation and risk communication, without any decision-making power. It provides opinions that the European Commission may or may not follow. This separation ensures a clear distinction between scientific expertise and political decision-making.26
In the U.S., however, the Food and Drug Administration (FDA) performs both risk assessment and regulatory functions, meaning it is responsible for analyzing the safety of additives as well as making decisions about their approval, restriction, or prohibition. Why is this a problem? This dual responsibility creates a conflict of interest risk, as the agency must both judge the danger of a substance and assume the economic and political consequences of its decision.
This model fundamentally differs from that of the European Union, where the EFSA provides independent expertise while the European Commission is responsible for decisions. In the U.S., the lack of separation between these roles makes the FDA more vulnerable to lobbying from industry and political pressure, which can delay or even prevent the withdrawal of certain additives suspected of posing health risks.
The FDA’s Lack of Methodology
Since 2010, European law has required the EFSA to reassess all food additives authorized before 2009.3 Currently, about 70% of additives have been reevaluated.27 In the United States, no such requirement exists. As a result, many additives approved more than 40 or 50 years ago, based on limited data, have never been reexamined in light of recent studies.
This difference in approach reflects two opposing visions. The European Union applies the precautionary principle, banning an additive as soon as scientific doubt exists about its safety. In contrast, the American system follows a reactive approach: a substance is considered “safe until proven otherwise”28 and bans occur only when a danger is definitively confirmed. Reassessments are generally triggered in response to petitions from public interest organizations rather than at the FDA’s own initiative.
Yellow 5 illustrates this problem. This artificial dye was authorized in the U.S. in 1969 based on a single FDA study conducted in 1964.29 That study did not assess any neurobehavioral parameters, even though the effects of Yellow 5 on the brain are a major concern today. Since its approval, 20 independent studies conducted between 1969 and 2022 have suggested a significant link between Yellow 5 and various harmful health effects, including neurobehavioral disorders in children, hyperactivity, allergic reactions, chronic diseases, and reduced fertility.30-33 Among these studies, two scientific reviews published in 2021 and 2022 have deemed the current safety threshold insufficient.34 Despite these concerning findings, which have persisted for nearly 50 years, the FDA has never reassessed this dye, which is heavily used in products widely consumed by children.
BHT, a widely used preservative, also highlights this regulatory inertia. When the FDA approved BHT in 1973, its evaluation already mentioned “uncertainties requiring further studies.”35 In 1979, a study by the National Cancer Institute raised concerns about its liver toxicity and potential carcinogenic effects. Yet, the FDA has never revised its assessment of this additive. Worse still, some criticisms present in the initial approval document were later removed after revision, without clear justification, raising questions about the transparency of the process.36
Furthermore, several indicators suggest that the FDA is less transparent and rigorous scientifically compared to other risk assessment agencies worldwide. While the FDA has published 15 documents37 outlining its methodology for evaluating the risks of food additives, this number is far lower than the data shared by other agencies. For comparison, the EFSA has published 37038 documents detailing its methods for assessing the toxicity of substances.
Among these methods, the EFSA establishes strict criteria for selecting studies used to identify the dangers of a substance.. A precise evaluation grid assigns a reliability score to studies, and only the most robust ones are considered. For example, an epidemiological study that fails to account for major risk factors such as alcohol or tobacco consumption is deemed biased. This approach serves as a crucial safeguard against arbitrary exclusion of studies by approval experts. To date, the FDA does not apply any precise methodology for selecting studies on additives. It relies only on a guide with very general recommendations for study selection, which are non-binding.40 A slightly more detailed methodology exists only for assessing carcinogenic risk.41
A 2018 study42 analyzing the evaluation methods of 63 agencies worldwide found that the FDA’s qualitative approach—relying on expert judgment without a scoring system or strict framework—can lead to divergent interpretations depending on the reviewers. By contrast, quantitative approaches like the EFSA’s, which use measurable criteria and an objective evaluation grid, ensure more reliable assessments.
Finally, the FDA generally conducts its evaluations based on average consumption levels without distinguishing between population groups. This method fails to identify specific risks for the most vulnerable groups, such as children, pregnant women, or people with higher exposure to additives. In contrast, agencies like the EFSA systematically consider different exposure scenarios to assess the impact on vulnerable populations. For instance, in its 1973 opinion on carrageenan, the FDA concluded there was no risk based on average consumption levels and set an acceptable daily intake (ADI) of 500 mg/kg/day.43 However, in its 2018 reassessment,44 the EFSA included different exposure scenarios, particularly considering high-consuming populations, as well as infants and young children who may consume medical products containing carrageenan. Due to uncertainties about certain effects, the EFSA set a provisional ADI of 75 mg/kg/day, adopting a much more cautious approach.
Europe also imposes strict limits on the presence of contaminants in additives, particularly toxic heavy metals such as arsenic, lead, mercury, and cadmium. The EFSA systematically incorporates these contaminants into its risk analyses. In the United States, while some specifications exist, they apply to only a minority of additives and are generally less protective. For example, the lead limit for sucrose esters of fatty acids—an emulsifier found in various products such as candy and biscuits—is five times higher than in Europe.45,46 Moreover, unlike European regulations, no limits are set for other heavy metals such as cadmium or mercury.
The Influence of Manufacturers on Regulation
The situation becomes even more concerning when industry directly interferes in the evaluation process. Under corporate pressure, the rules for approving additives have been progressively loosened, significantly reducing the FDA’s oversight of additive regulation. Before 1997, the FDA was directly responsible for evaluating the safety of substances added to food. To introduce a new ingredient, a company had to submit a dossier with scientific data proving its safety. The FDA would then conduct a thorough analysis before deciding on its approval, a process that could take several years.47
In 1997, following industry pressure to speed up approval times, the FDA modified the approval process for GRAS (Generally Recognized As Safe) substances. This status, created in 1958, originally applied only to ingredients such as salt, pepper, or vinegar, whose safety was well-established through decades of use and validated by scientific consensus.48
With the new process, manufacturers gained the power to self-declare a substance as GRAS, merely notifying the FDA. Instead of conducting an in-depth evaluation, the agency now simply reviews the information provided by the company and issues a non-binding opinion.49 Before giving a negative response, the FDA warns the manufacturer, who usually withdraws their notification to avoid receiving an official rejection.50
This change drastically weakened oversight mechanisms. Since 1997, manufacturers can fund their own studies, which are rarely accessible to the public and not subject to peer review—a critical process for ensuring the reliability of scientific research. A 2013 study found that between 1997 and 2013, over 60% of GRAS substances were approved based on industry-funded research or studies conducted by scientists affiliated with manufacturers.41
The problem worsened in 2016 when GRAS notification to the FDA became entirely optional. Manufacturers can now introduce new GRAS substances to the market without even informing the agency. Furthermore, while the GRAS status is supposed to be validated by experts, regulations do not specify any requirements for their qualifications. The FDA itself states that “any person, generally a food or ingredient manufacturer, who has determined that the use of an ingredient meets GRAS standards can notify the FDA through the GRAS notification program.”41 In other words, companies determine the safety of their own additives, with no strict oversight or independent supervision. A study conducted in 2023 revealed that between 2015 and 2020, 30% of GRAS declarations were based solely on the opinion of experts internal to the company promoting the additive. This system, which has gradually become more lenient over the years, increasingly and openly prioritizes the economic interests of industry over public health safety.
Since 2000, nearly 99% of new substances added to food have been approved under the GRAS status by companies themselves, according to an investigation by the Environmental Working Group (EWG).52 Only 1% were submitted to the FDA for approval, typically when the additive manufacturer was not the producer of the final product, as FDA validation primarily serves to reassure food manufacturers.
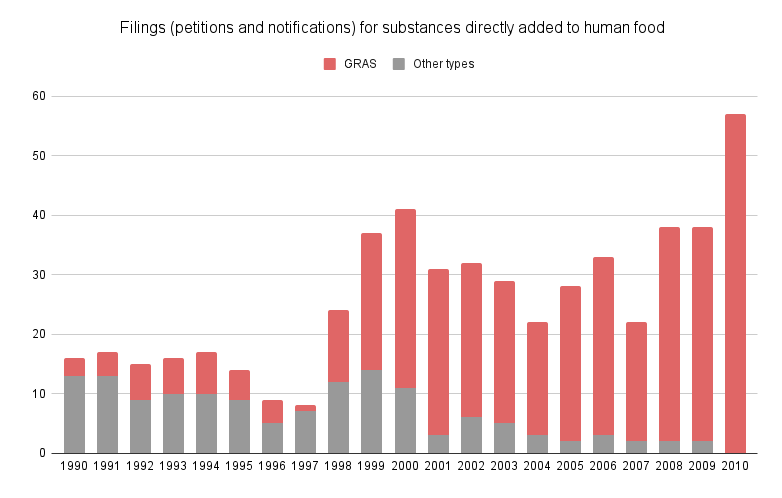
Source: (Neltner et al, 2013)53
The question of conflicts of interest in food additive evaluation in the United States is a major concern. A 2013 study, based on conflict-of-interest criteria defined by the Institute of Medicine, revealed the pervasive financial conflicts of interest in GRAS determinations. Out of 451 GRAS notifications analyzed, none of the evaluations were conducted by an independent panel designated by a third-party organization. Instead, they were performed by an employee of the manufacturer in 22.4% of cases, an employee of a consulting firm chosen by the manufacturer in 13.3% of cases, or an expert panel selected either by the manufacturer or by a consulting firm paid by the manufacturer in 64.3% of cases.53
These panels are also highly limited, with an average of only 3.5 members. A 2023 study analyzing over 400 GRAS notifications found that the same seven experts appeared in more than half of the panels and frequently worked together.42 Rather than forming independent groups for each evaluation, as the FDA recommends, the same experts are consistently used—many of whom work for consulting firms funded by the food industry.
Aspartame is a perfect example of how conflicts of interest influence public health decisions. Developed by the company Searle in the 1970s, this artificial sweetener was initially rejected by the FDA due to a lack of evidence regarding its safety.54 An internal FDA report from 1980 raised concerns about its potential carcinogenic effects.55 However, the election of Ronald Reagan in 1981 changed the situation. Close to Searle’s CEO, Reagan appointed a new FDA director who quickly approved aspartame based on a controversial study funded by Ajinomoto, a manufacturer of aspartame.56 This study, which was never published in a peer-reviewed journal and contained numerous methodological flaws, did not meet the FDA’s own scientific standards. Notably, six FDA officials involved in the approval process later joined companies associated with aspartame production.57
Conclusion
Despite this rather bleak picture, some encouraging signs suggest a possibility for change. Two dynamics could push the U.S. system toward better consumer protection: citizen mobilization and initiatives from pioneering states.
First, consumer advocacy and public interest organizations play a key role in a system that operates reactively rather than preventively. Petitions and grassroots mobilization efforts have already proven effective in forcing the reassessment of certain additives. For example, in 2018, the FDA banned six synthetic flavorings in response to two petitions filed in 2015 by several public health and environmental organizations, which denounced their carcinogenic nature.59
Another encouraging example came in 2020 when public health associations submitted a citizen petition calling for an overhaul of risk assessment methods, emphasizing the cumulative effect of additives.60 This public pressure contributed to the emergence of two legislative proposals: in 2021, the Food Chemical Reassessment Act61 established a list of ten priority substances to be reexamined, while in 2024, the Toxic Free Food Act62 proposed strengthening transparency in the GRAS approval process.
Additionally, some pioneering states, such as California and New York, are creating positive momentum that pushes the federal system to evolve. By adopting stricter measures than the FDA, they demonstrate that more protective regulations are possible and encourage other states to follow suit. California, in particular, has played a leading role. In 2023, it banned the food dye Red 3, suspected of being carcinogenic due to its effects on the thyroid, triggering a chain reaction.63 Several states announced that they would follow this ban, and in February 2025, the FDA finally prohibited this additive at the federal level.64
These developments show that even in a system heavily influenced by industry, citizen pressure and local initiatives can create a decisive push for change. Awareness is growing, and with it, the hope that U.S. regulations will gradually evolve toward a more protective framework for public health.
- ¹ Julie Chapon (2025). Yuka study on additive quantity in food products. Available on: https://yuka.io/en/yuka-study-additive-quantity-food-products/
- ² International Agency for Research on Cancer (2010). Carbon Black, Titanium Dioxide, and Talc. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 93. Retrieved from : https://publications.iarc.fr/111
- ³ Commission Regulation (EU) 2022/63 of 14 January 2022 amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the food additive titanium dioxide (E 171). Retrieved from : https://eur-lex.europa.eu/eli/reg/2022/63/oj/eng
- ⁴ European Food Safety Authority (2021). Safety assessment of titanium dioxide (E171) as a food additive. Retrieved from : https://www.efsa.europa.eu/fr/efsajournal/pub/6585
- ⁵ Veille Nano (2023). Along with Turkey, at least 36 countries have already said no to E171 in food. Retrieved from : https://veillenanos.fr/en/ban-e171-turkey-world/
- ⁶ European Parliament and of the Council (2006). Directive 2006/52/EC of 5 July 2006 amending Directive 95/2/EC on food additives other than colours and sweeteners and Directive 94/35/EC on sweeteners for use in foodstuffs. Retrieved from : https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32006L0052
- ⁷ European Food Safety Authority (EFSA) (2004). Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) related to para hydroxybenzoates (E 214-219). EFSA Journal, 2(9), 1-26. https://doi.org/10.2903/j.efsa.2004.83
- ⁸U.S. Department of Agriculture (USDA) (2024). China: Usage Standard for Food Additives Finalized. May 6, 2024, Attaché Report (GAIN), CH2024-0059. Retrieved from : https://www.fas.usda.gov/data/china-usage-standard-food-additives-finalized
- ⁹U.S. Food and Drug Administration (FDA) (1984). 21 CFR 184.1670 – Propylparaben. eCFR. Retrieved from: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-184/subpart-B/section-184.1670
- ¹⁰ California State Assembly (2023). Assembly Bill No. 418: The California Food Safety Act. Chapter 328, Statutes of 2023. Retrieved from https://leginfo.legislature.ca.gov/faces/billNavClient.xhtml?bill_id=202320240AB418
- ¹¹ https://yuka.io/en/yuka-study-food-additive-approval-european-union/
- ¹² Wang, Z.; Wei, X.; Yang, J.; Suo, J.; Chen, J.; Liu, X.; Zhao, X (2016). Chronic Exposure to Aluminum and Risk of Alzheimer’s Disease: A Meta-Analysis. Neurosci. Lett. 2016, 610, 200–206.
- ¹³ Mold M, Umar D, King A, Exley C (2017). Aluminium in brain tissue in autism. J Trace Elem Med Biol. 2018 Mar;46:76-82. doi: 10.1016/j.jtemb.2017.11.012. Epub 2017 Nov 26. PMID: 29413113.
- ¹⁴ Mold M, Cottle J, Exley C. (2019). Aluminium in Brain Tissue in Epilepsy: A Case Report from Camelford. Int J Environ Res Public Health. 2019 Jun 16;16(12):2129. doi: 10.3390/ijerph16122129. PMID: 31208130; PMCID: PMC6616903.
- ¹⁵ German Federal Institute for Risk Assessment (BfR) (2019). Reducing aluminium intake can minimise potential health risks. https://www.bfr.bund.de/cm/349/reducing-aluminium-intake-can-minimise-potential-health-risks.pdf
- ¹⁶ Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. Official Journal of the European Union, L 354, 16-33. Retrieved from https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32008R1333
- ¹⁷ U.S. Food and Drug Administration (FDA) (2016). Select Committee on GRAS Substances (SCOGS) Opinion: Aluminum salts. FDA. Retrieved from https://wayback.archive-it.org/7993/20171031060456/https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/SCOGS/ucm260848.htm
- ¹⁸ https://yuka.io/en/yuka-study-food-additive-usage-restrictions/
- ¹⁹ Nigg JT, Lewis K, Edinger T, Falk M. (2012) Meta-analysis of attention-deficit/hyperactivity disorder or attention-deficit/hyperactivity disorder symptoms, restriction diet, and synthetic food color additives. J Am Acad Child Adolesc Psychiatry Jan;51(1):86-97.e8. doi: 10.1016/j.jaac.2011.10.015. PMID: 22176942; PMCID: PMC4321798.
- ²⁰ Motwadie, M.E., Hashem, M.M., Abo-El-Sooud, K., Abd-Elhakim, Y.M., El-Metwally, A. E., Ali, H.A., (2021). Modulation of immune functions, inflammatory response, and cytokine production following long-term oral exposure to three food additives; thiabendazole, monosodium glutamate, and brilliant blue in rats. Int. Immunopharm. 98 (March), 107902 https://doi.org/10.1016/j. intimp.2021.107902.
- ²¹ EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) (2010); Scientific Opinion on the re-evaluation of Brilliant Blue FCF (E 133) as a food additive. EFSA Journal 2010; 8(11):1853. [36 pp.]. doi:10.2903/j.efsa.2010.1853
- ²² U.S. Food and Drug Administration (FDA) (2022). Summary of Color Additives for Use in the United States in Foods, Drugs, Cosmetics, and Medical Devices. Retrieved from : https://www.fda.gov/industry/color-additives/summary-color-additives-use-united-states-foods-drugs-cosmetics-and-medical-devices
- ²³ Stevens LJ, Burgess JR, Stochelski MA, Kuczek T (2015). Amounts of artificial food dyes and added sugars in foods and sweets commonly consumed by children. Clin Pediatr (Phila). 2015 Apr;54(4):309-21. Retrieved from: https://pubmed.ncbi.nlm.nih.gov/24764054/
- ²⁴ U.S. Food and Drug Administration (FDA) (1984). 21 CFR 172.175 – Sodium nitrite. eCFR. Retrieved from: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-172/subpart-B/section-172.175
- ²⁵ International Agency for Research on Cancer (IARC) (2010). Ingested Nitrate and Nitrite, and Cyanobacterial Peptide Toxins IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 94. Available on: https://publications.iarc.fr/112
- ²⁶ European Food Safety Authority (EFSA) (2014). Risk assessment vs risk management: What’s the difference? [Infographic]. EFSA. Retrieved from https://www.efsa.europa.eu/en/discover/infographics/risk-assessment-vs-risk-management-whats-difference
- ²⁷ European Food Safety Authority (EFSA) (2025). Food additives. Retrieved from: https://www.efsa.europa.eu/en/topics/topic/food-additives
- ²⁸ The weight of evidence (2010). Nature 464, 1103–1104 . https://doi.org/10.1038/4641103b
- ²⁹ Davis, K. J., Fitzhugh, O. G., & Nelson, A. A. (1964). Chronic rat and dog toxicity studies on Tartrazine.Toxicol Appl Pharmacol. 1964 Sep;6:621-6. doi: 10.1016/0041-008x(64)90095-x. PMID: 14217004.
- ³⁰ Barciela, P., Perez-Vazquez, A., & Prieto, M. A. (2023). Azo dyes in the food industry: Features, classification, toxicity, alternatives, and regulation. Food and Chemical Toxicology, 178. https://doi.org/10.1016/j.fct.2023.113935
- ³¹ Office of Environmental Health Hazard Assessment (OEHHA) (2021). Health Effects Assessment: Potential Neurobehavioral Effects of Synthetic Food Dyes in Children. California Environmental Protection Agency.
- ³² Nigg JT, Lewis K, Edinger T, Falk M (2012). Meta-analysis of attention-deficit/hyperactivity disorder or attention-deficit/hyperactivity disorder symptoms, restriction diet, and synthetic food color additives. J Am Acad Child Adolesc Psychiatry. 2012 Jan;51(1):86-97.e8. doi: 10.1016/j.jaac.2011.10.015. PMID: 22176942; PMCID: PMC4321798.
- ³³ Arnold et al. (2012). Artificial food colors and attention-deficit/hyperactivity symptoms: conclusions to dye for. Neurotherapeutics, 9(3), 599-609.
- ³⁴ Miller, M.D., Steinmaus, C., Golub, M.S. et al (2022). Potential impacts of synthetic food dyes on activity and attention in children: a review of the human and animal evidence. Environ Health 21, 45. 2022. https://doi.org/10.1186/s12940-022-00849-9
- ³⁵ U.S. Food and Drug Administration (FDA) Select Committee on GRAS Substances (SCOGS) Opinion: Butylated Hydroxytoluene (BHT) (1973). FDA. Retrieved from http://wayback.archive-it.org/7993/20171031063105/https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/SCOGS/ucm260875.htm
- ³⁶ National Toxicology Program (NTP) (1979). Bioassay of Butylated Hydroxytoluene (BHT) for Possible Carcinogenicity (Technical Report No. 150). U.S. Department of Health, Education, and Welfare, Public Health Service, National Institutes of Health. Retrieved from https://ntp.niehs.nih.gov/sites/default/files/ntp/htdocs/lt_rpts/tr150.pdf
- ³⁷ U.S. Food and Drug Administration (FDA) (2025). Guidance Documents & Regulatory Information by Topic (Food and Dietary Supplements). Available on: https://www.fda.gov/food/guidance-regulation-food-and-dietary-supplements/guidance-documents-regulatory-information-topic-food-and-dietary-supplements
- ³⁸ European Food Safety Authority (EFSA) (2025). Guidance and other assessment methodology documents. Available on: https://www.efsa.europa.eu/en/methodology/guidance
- ³⁹ European Food Safety Authority (EFSA) (2020). Draft for internal testing Scientific Committee guidance on appraising and integrating evidence from epidemiological studies for use in EFSA's scientific assessments. Available on: https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2020.6221
- ⁴⁰ U.S. Food and Drug Administration (FDA) (2007). Guidance for Industry and Other Stakeholders: Redbook 2000. Toxicological Principles for the Safety Assessment of Food Ingredients. July 2007. Available on: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-and-other-stakeholders-redbook-2000#TOC
- ⁴¹ U.S. Food and Drug Administration (FDA) (2009). Guidance for Industry: Evidence-Based Review System for the Scientific Evaluation of Health Claims. January 2009. Available on: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-evidence-based-review-system-scientific-evaluation-health-claims#system
- ⁴² Pierre Martin, Claire Bladier, Bette Meek, Olivier Bruyere, Eve Feinblatt, Mathilde Touvier, Laurence Watier, and David Makowski (2018). Weight of Evidence for Hazard Identification: A Critical Review of the Literature. Environmental Health Perspectives, Volume 126, Issue 7, CID: 076001. https://ehp.niehs.nih.gov/doi/10.1289/EHP3067
- ⁴³ U.S. Food and Drug Administration (FDA) (1973). Evaluation of the Health aspects of Carrageenan as a food ingredient. Retrieved from : https://ntrl.ntis.gov/NTRL/dashboard/searchResults.xhtml?searchQuery=PB266877
- ⁴⁴ European Food Safety Authority (EFSA) (2018). Re‐evaluation of carrageenan (E 407) and processed Eucheuma seaweed (E 407a) as food additives. Retrieved from : https://www.efsa.europa.eu/en/efsajournal/pub/5238
- ⁴⁵ Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council. Official Journal of the European Union, L 83, 1-295.
- ⁴⁶ U.S. Food and Drug Administration (FDA) (2023). 21 CFR 172.859 – Sucrose fatty acid esters. eCFR. Retrieved from https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-172/subpart-I/section-172.859
- ⁴⁷ Neltner, T.G., Kulkarni, N.R., Alger, H.M., Maffini, M.V., Bongard, E.D., Fortin, N.D. and Olson, E.D. (2011). Navigating the U.S. Food Additive Regulatory Program. Comprehensive Reviews in Food Science and Food Safety, 10: 342-368. https://doi.org/10.1111/j.1541-4337.2011.00166.x
- ⁴⁸ U.S. Food and Drug Administration (FDA) (2018). FDA’s approach to the GRAS provision: History and processes. FDA. Retrieved from: https://www.fda.gov/food/generally-recognized-safe-gras/fdas-approach-gras-provision-history-processes
- ⁴⁹ U.S. Food and Drug Administration (FDA) (2018). History of the GRAS list and SCOGS reviews. FDA. Retrieved from: https://www.fda.gov/food/gras-substances-scogs-database/history-gras-list-and-scogs-reviews
- ⁵⁰ Neltner, T. G., Alger, H. M., Leonard, J. E., & Maffini, M. v. (2013). Data gaps in toxicity testing of chemicals allowed in food in the United States. Reproductive Toxicology, 42, 85–94. https://doi.org/10.1016/j.reprotox.2013.07.023
- ⁵¹ Matouskova, K., Neltner, T. G., & Maffini, M. V. (2023). Out of balance: conflicts of interest persist in food chemicals determined to be generally recognized as safe. Environmental Health, 22(59). https://doi.org/10.1186/s12940-023-01004-8
- ⁵² Environmental Working Group (EWG). (2022). EWG analysis: Almost all new food chemicals greenlighted by industry, not FDA. EWG. Retrieved from https://www.ewg.org/news-insights/news/2022/04/ewg-analysis-almost-all-new-food-chemicals-greenlighted-industry-not-fda
- ⁵³ Neltner TG, Alger HM, O'Reilly JT, Krimsky S, Bero LA, Maffini MV (2013). Conflicts of interest in approvals of additives to food determined to be generally recognized as safe: out of balance. JAMA Intern Med. (2013) Dec 9-23;173(22):2032-6. doi: 10.1001/jamainternmed.2013.10559. PMID: 23925593.
- ⁵⁴ Millstone, E.P., Dawson, E. (2019). EFSA’s toxicological assessment of aspartame: was it even-handedly trying to identify possible unreliable positives and unreliable negatives?. Arch Public Health 77, 34, 2019. https://doi.org/10.1186/s13690-019-0355-z
- ⁵⁵ U.S. Food and Drug Administration (FDA) (1980), Aspartame: Decision of the Public Board of Inquiry, Department of Health and Human Services, Food and Drug Administration, docket no. 75F-0355, 30th September 1980.
- ⁵⁶ Ishii H. Incidence of brain tumors in rats fed aspartame. Toxicol Lett. 1981 Mar;7(6):433-7. doi: 10.1016/0378-4274(81)90089-8. PMID: 7245229.
- ⁵⁷ Morgan Sykes (2015). The aspartame controversy of 1981 The Hidden Truth Behind the Not-So-Sweet Artificial Sweetener. Available on: https://vtuhr.org/articles/33/files/submission/proof/33-1-60-1-10-20171116.pdf
- ⁵⁸ Center for Science in the Public Interest, Natural Resources Defense Council, Center for Food Safety, Consumers Union, Improving Kids’ Environment, Center for Environmental Health, Environmental Working Group, & James Huff (2015). Revised Food Additive Petition FAP 5A4810: Request to remove FDA’s approval of seven synthetic flavors due to carcinogenicity. Submitted to the U.S. Food and Drug Administration. Retrieved from FDA Docket FDA-2015-F-4317-0003.
- ⁵⁹ U.S. Food and Drug Administration (FDA) (2018). FDA removes 7 synthetic flavoring substances from food additives list. FDA. Retrieved from https://www.fda.gov/food/hfp-constituent-updates/fda-removes-7-synthetic-flavoring-substances-food-additives-list
- ⁶⁰ Environmental Defense Fund, et al. (2020).Cumulative Effects Citizen Petition to FDA. Division of Dockets Management, Food and Drug Administration, Department of Health and Human Services, September 23, 2020.
- ⁶¹ U.S. Congress (2021). H.R.4694 – Food Chemical Reassessment Act of 2021. 117th Congress. Retrieved from https://www.congress.gov/bill/117th-congress/house-bill/4694
- ⁶² U.S. Congress (2024). H.R.9817 – Toxic Free Food Act of 2024. 118th Congress. Retrieved from https://www.congress.gov/bill/118th-congress/house-bill/9817
- ⁶³ California State Assembly (2023). Assembly Bill No. 418: The California Food Safety Act. Chapter 328, Statutes of 2023. Retrieved from https://leginfo.legislature.ca.gov/faces/billNavClient.xhtml?bill_id=202320240AB418
- ⁶⁴ U.S. Food and Drug Administration (FDA) (2023). FDA to revoke authorization for the use of Red No. 3 in food and ingested drugs. FDA. Retrieved from https://www.fda.gov/food/hfp-constituent-updates/fda-revoke-authorization-use-red-no-3-food-and-ingested-drugs




